Glycotype Analysis Strategy of Protein Drugs
Protein drugs such as therapeutic monoclonal antibodies (mAbs) are glycoproteins produced by living cell systems. The glycan moieties attached to the proteins can directly affect protein stability, bioactivity, and immunogenicity. Therefore, glycan variants of a glycoprotein product must be adequately analyzed and controlled to ensure product quality. With the development of detection technologies, various strategies and instruments are applied in glycotype analysis of protein drugs. As a world-leading services provider in the field of protein engineering, Creative Biolabs has developed a comprehensive and established glycotype analysis platform to clarify the oligosaccharide chain, glycosylation site, and glycopeptide analysis. Based on lots of measurement technologies including Lectin Microarray, MS, LC-ESI-MS, MALDI-TOF MS, HPLC, HPAEC-PAD, RP-HPLC, UHPLC/FLD/Q-TOF, FTIR, NMR, SPRi, TLC, Flow Cytometry, GC-MS, Imaging Mass Spectrometry, and Glycobiology Microarray. Our experienced research group can submit a comprehensive analysis report on glycotype analysis of protein drugs rapidly and accurately to our clients all over the world.
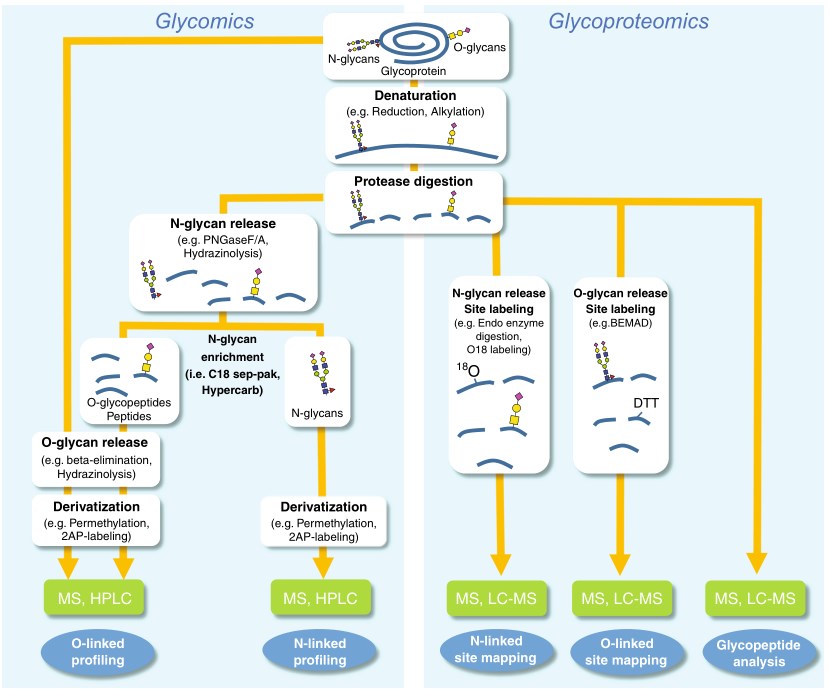 Fig.1 General workflows for glycomics and glycoproteomics analysis.1, 2
Fig.1 General workflows for glycomics and glycoproteomics analysis.1, 2
Introduction of Glycotype Analysis Strategy of Protein Drugs
Protein glycosylation is a complex post-translational modification involving the attachment of glycans at specific sites on a protein, most commonly at Asn (N-linked) or Ser/Thr (O-linked) residues. The multiple levels of glycan heterogeneity pose a daunting analytical challenge. In the development of protein drugs, glycan analysis usually involves the use of complementary methods for assessing specific glycosylation attributes, including glycosylation site, glycan structure, and sugar abundance.
The structural analysis of protein glycosylation is generally performed with or without the release of the glycan modification from the glycoproteins: the former approach is termed “glycomics”, and the latter is termed “glycoproteomics”. Releasing the glycans in the glycomics approach enables direct introduction into MS instrument and allows higher-dimensional MS analysis to obtain the oligosaccharide chain. However, once the glycan is released from the protein, the site-specific information, such as the attachment site and occupancy rates, is lost. Therefore, in the glycoproteomics approach, the glycans are not released, and the glycan-peptide bonds are carefully kept intact to obtain information about glycosylation sites and site occupancies.
What Can We Do?
As the basic analysis method in glycotype analysis, some technologies including MS, HPLC, GC-MS, NMR, and HPAEC-PAD are applied in oligosaccharide chain analysis to show the monosaccharide types, abundance, and linkage of complex oligosaccharide chains.
Glycosylation site analysis reveals the potential glycosylation sites that are occupied and eventually defines the structure of specific protein drug molecules. Moreover, this information is very useful for data analysis in the subsequent glycopeptide analysis.
After protease digestion, the mixture of N- and O-linked glycopeptides and peptides can be analyzed directly by LC-MS. Glycopeptide analysis can obtain several key fragments characteristic of the overall glycopeptide structure and elucidate the glycopeptide structure furtherly.
With the wide application of glycoproteins in protein drugs development, the field of analytical glycobiology has evolved substantially. Therefore, Creative Biolabs launchs a series of one-stop and customized glycotype analysis services to support your research. Do not hesitate to contact us for more details.
References
-
Shajahan, Asif, et al. "Glycomic and glycoproteomic analysis of glycoproteins—a tutorial." Analytical and bioanalytical chemistry 409 (2017): 4483-4505.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 General workflows for glycomics and glycoproteomics analysis.1, 2
Fig.1 General workflows for glycomics and glycoproteomics analysis.1, 2
