Knockout and Knockin by Precision Genome Editing
As a leading provider of glycoprotein related services, Creative Biolabs can help you establish customized cell line for glycoengineering by precision genome editing. With over a decade of experience and the state-of-the-art technical platforms, we can totally meet your project requirements and budgets in the gene knockout and knockin services.
Gene Knockout and Knockin
Gene knockout is a genetic technique that makes one or more genes inoperative in living organism. Knockout organisms are used to study the gene function, usually by investigating the deficiency effect of the gene. In post-translational modifications, studies have reported the knockin of human N-glycan-modifying enzymes to obtain non-immunogenic and/or ‘humanized’ N-glycans on glycosylated plant-made therapeutics. Gene knockout/knockin is a powerful tool for determining gene function or permanently modifying the phenotypic characteristics of a cell.
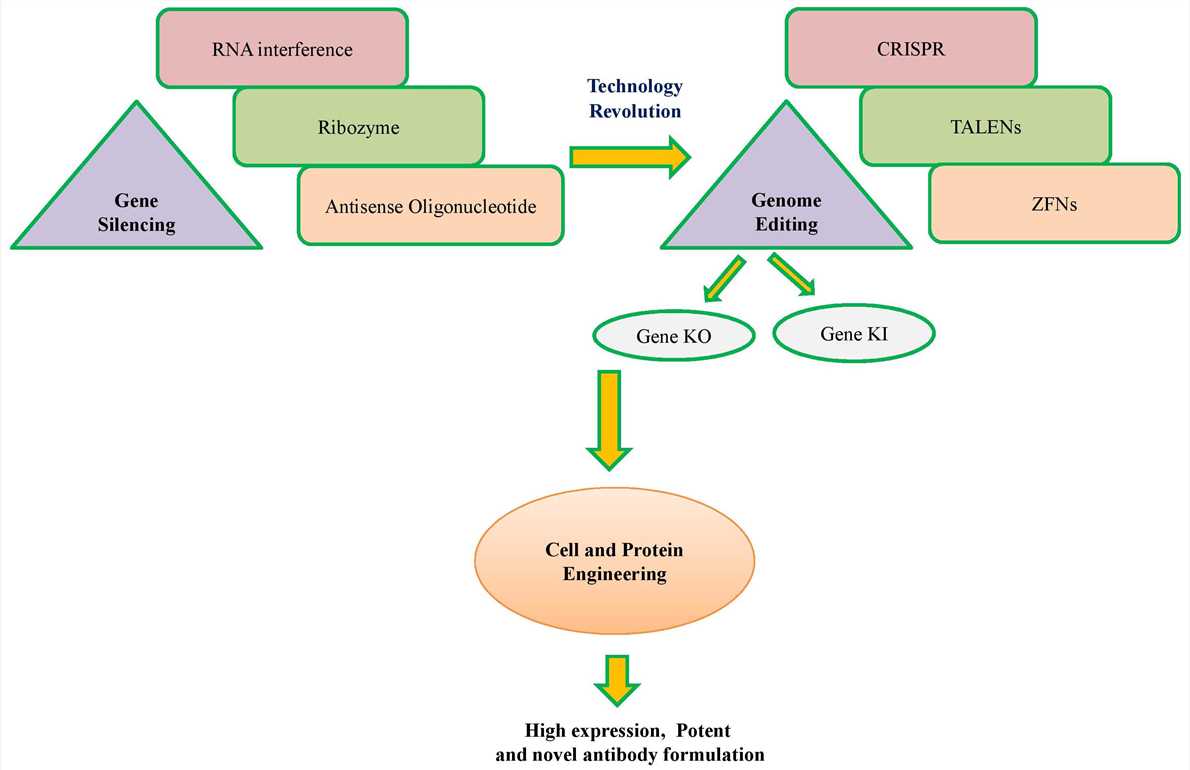 Fig.1 Technological developments in cell engineering.1, 5
Fig.1 Technological developments in cell engineering.1, 5
Applications in Glycoengineering
Many experimental cases demonstrate the effectiveness of gene knockout/knockin or combinational genetic manipulation in glycosylation engineering.
-
Chinese Hamster Ovary (CHO) Cells
CHO cells were successfully engineered to produce rhEPO containing almost exclusively α2,6-sialylation by knockout of st3gal4/6 genes and knockin of st6gal-I gene, which resulted in considerable increase of α2,6 sialylation. Homogeneous bi-antennary N-glycans capped by α-2,6-NeuA were produced by additional knockout of mgat4A/4B/5 genes. Double knockout of fut8 and B4galt1 genes in CHO cells expressing a recombinant IgG1 resulted in homogeneous biantennary N-glycans lacking Fuc, containing minor amounts of Gal. CHO glutamine synthetase (GS)-knock out cell lines were developed. Fut8 knockout CHO cell lines have been established and one is currently available on the market for glycoprotein productions. CRISPR-Cas9 system has been used to knockout GDP-fucose transporter (GFT) gene in CHO cell line for the production of various afucosylated mAbs and Fc-fusion proteins. Other strategies include knockout of CMP-Neu5Ac hydroxylase (CMAH), α1,3-galactosyltransferase (α1,3-GalT), and so forth.
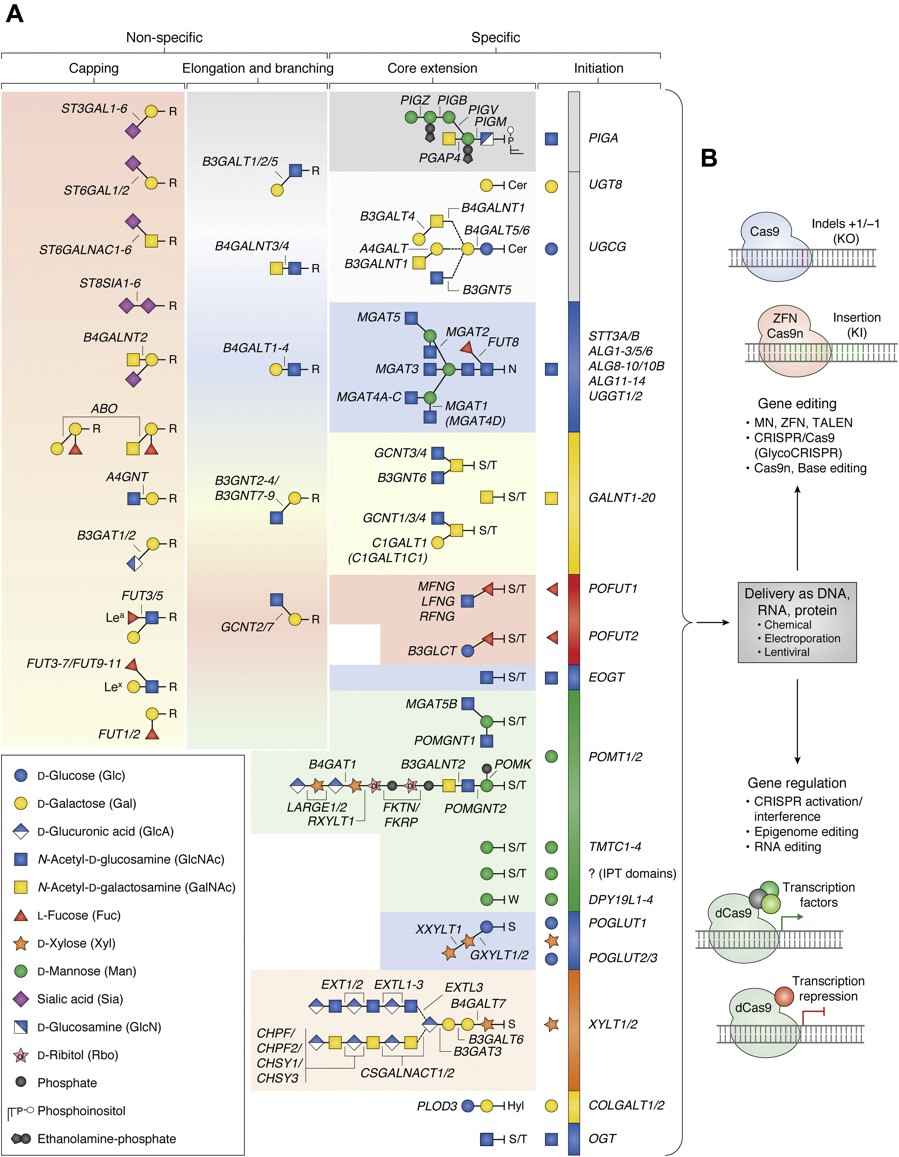 Fig.2 Some glycoengineering strategies and gene editing tools.2, 5
Fig.2 Some glycoengineering strategies and gene editing tools.2, 5
Knockout α1,6-mannosyltransferase (Och1p) in yeast has been established. Double knockout of both STE13 and DAP2 facilitating the production of recombinant glycoproteins with their intact native sequence in yeast. Transfer and elongation of O-linked mannose can be avoided by knockout of protein O-mannosyltransferases (PMTs) in yeast.
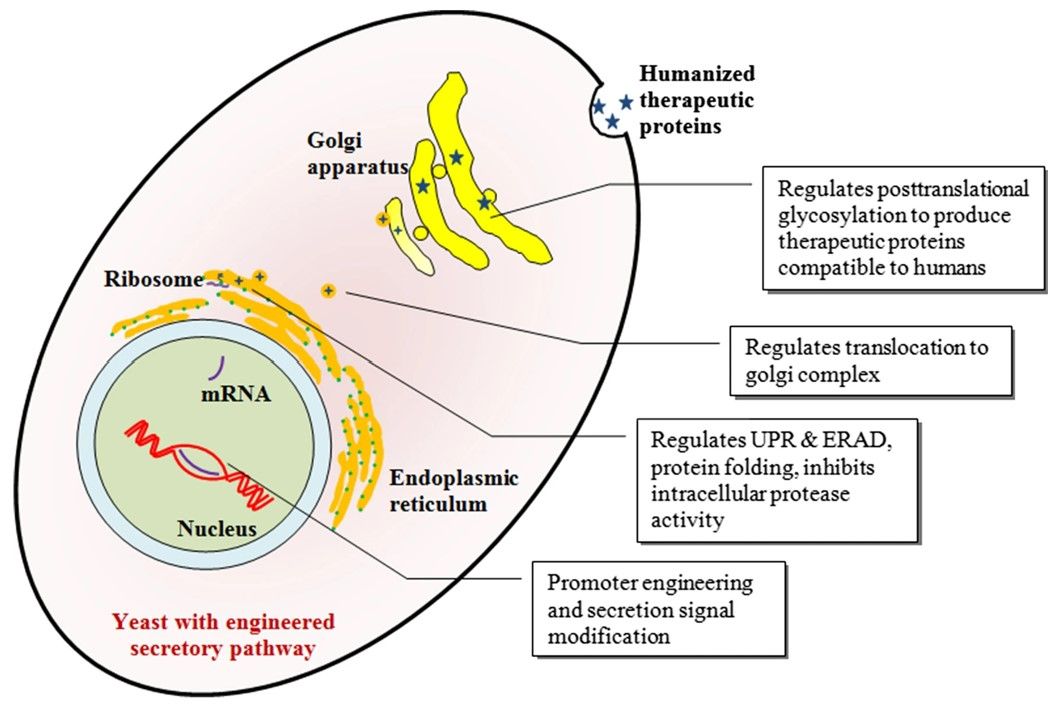 Fig.3 Modifying yeast through strategies such as gene knockout.3, 5
Fig.3 Modifying yeast through strategies such as gene knockout.3, 5
In plants, a BY2 cell line devoid of plants specific xylose and fucose by knocking out of the XylT and the FucT genes has been established. The glycoengineered BY2 cell lines provide a valuable platform for producing potent biopharmaceutical products that can be similar to the mammalian proteins. Similarly, knockout of other plant-specific N-glycan maturation enzymes, such as α1,3-fucosyltransferases (α1,3-FTs) and β1,2- xylosyltransferase (β1,2-XT), or the knockin of human N-glycan-modifying enzymes have been proved to be successful in plant cells. Unwanted plant-specific modifications also can be eliminated by knockout of the responsible enzymes like prolyl 4-hydroxylases (P4H).
In insect cells, knockout of core α1,3-fucosyltransferase (FUT) and b-N-acetylglucosaminidase (β-hex) was reported. CRISPR-Cas9 system has also been reported for editing fdl gene in S2 cells, resulting in S2 cells produced partially elongated, mammalian-type complex N-glycans.
Features of Our Knockout/Knockin Services
-
Strategies for gene knockout/knockin: CRISPR/Cas9 system, transcription activator-like effector nucleases, and homologous recombination technology.
-
Complete project report
-
High quality and fast delivery
-
Best after-sale service
Creative Biolabs offers a range of gene manipulation solutions by gene knockout and gene knockin techniques to fit your demands. Scientists in our laboratory have accumulated extensive experience in precision genome editing for site-specific gene manipulation. Please feel free to contact us for more details.
Published data
Knock-in and knock-out are effective strategies to modify the glycosylation pathway in CHO cells. In this study, researchers used clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) to knock down relevant genes in CHO. A series of CHO cell lines with different abilities to produce lysosomal enzymes were eventually constructed by knockdown. These CHO cell lines have all the key glycan features known to affect cycling time and cellular uptake. Not only that, but the researchers also utilized a mouse model of Fabry disease to show us how different glycosylated forms of alpha-galactosidase A (GLA) differently target the heart, spleen, kidney, and liver. This study provides data to support the study of treatments for rare congenital lysosomal enzyme deficiencies through a comprehensive analysis, as well as a demonstration of how we can effectively design and modify glycosylation pathways in CHO cells.
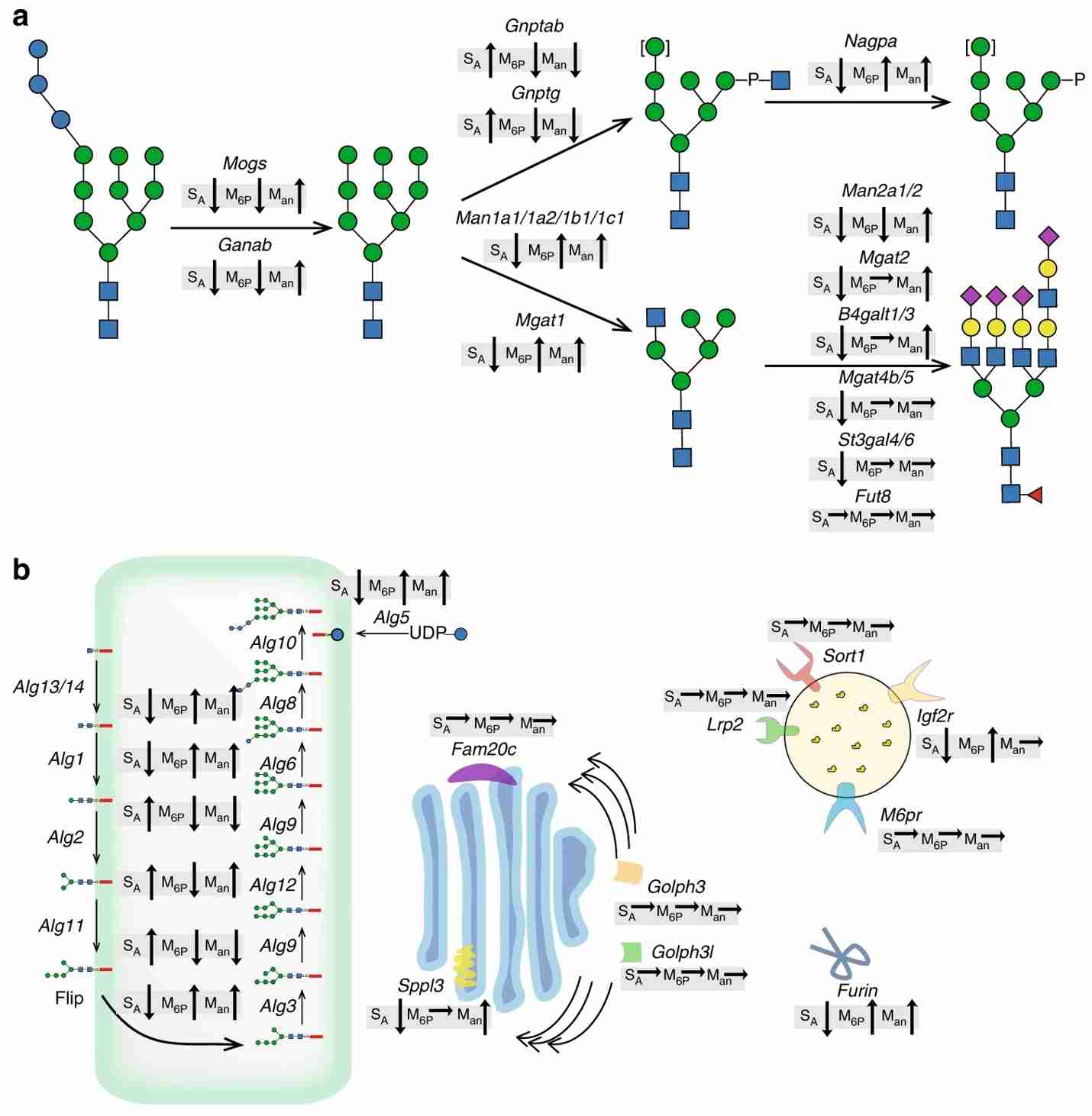 Fig.4 Impact of gene editing on overall trends in GLA N-glycosylation.4, 5
Fig.4 Impact of gene editing on overall trends in GLA N-glycosylation.4, 5
FAQs
Q1: What techniques are available for knock-in or knock-out of genes?
A1: Precision genome editing technologies enable stable genetic changes in cellular glycosylation mechanisms. The development of these technologies provides a wide range of opportunities for knock-in or knock-out to modulate glycosylation pathways.
-
CRISPR/Cas9: The CRISPR/Cas9 system is capable of reliably replacing, deleting, or inserting precise genomic sequences, and has been successfully used in a variety of organisms, such as yeast, mouse, and mammalian cell lines. Used in conjunction with two sgRNAs, this system is a simple and effective method for manipulating large genomic DNA fragments.
-
Transcription activator-like effector nucleases (TALENs): TALENs technology has become a powerful choice for precise genome editing due to its ease of operation and high gene targeting specificity. Currently, TALENs have been applied to genome editing in different species, such as mice, yeast, rice, and maize. It can recognize the target gene more specifically and is not affected by its upstream and downstream sequences.
-
Other precision genome editing technologies
Q2: What applications have gene insertion or knockout been used for?
A2: Insertion or knockout is used for targeted modification of cell lines, study of glycosylation substrates, and production of various therapeutic glycoproteins. In addition, effective gene insertion or knockout also accelerates the generation of animal models, which facilitates the study of disease mechanisms and the search for therapeutic targets.
Customer Review
Promoting Glycoprotein Production Through Knockdown
"We recently turned to Creative Biolabs to help us design glycosylation pathways to produce specific glycan structures. They used advanced gene editing techniques to help us precisely knock out genes to facilitate the production of recombinant glycoproteins. Not only were they responsive, but the accuracy and efficiency of their services were also very high. It was a very soothing experience working with them."
Highly Efficient Gene Editing Program
"There was no doubt that Creative Biolabs had a high degree of precision in this area of gene knockout or gene knock-in. After understanding our needs, they provided highly efficient, successful, and thorough gene editing solutions to solve our problems such as high off-target rates. Their team was also readily available to answer any questions we had throughout the process. They delivered on their promise of client-centered, high-quality service."
References
-
Dangi, Arun K., et al. "Cell line techniques and gene editing tools for antibody production: a review." Frontiers in pharmacology 9 (2018): 379411.
-
Narimatsu, Yoshiki, et al. "Genetic glycoengineering in mammalian cells." Journal of Biological Chemistry 296 (2021).
-
Madhavan, Aravind, et al. "Customized yeast cell factories for biopharmaceuticals: from cell engineering to process scale up." Microbial Cell Factories 20.1 (2021): 124.
-
Tian, Weihua, et al. "The glycosylation design space for recombinant lysosomal replacement enzymes produced in CHO cells." Nature communications 10.1 (2019): 1785.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 Technological developments in cell engineering.1, 5
Fig.1 Technological developments in cell engineering.1, 5
 Fig.2 Some glycoengineering strategies and gene editing tools.2, 5
Fig.2 Some glycoengineering strategies and gene editing tools.2, 5
 Fig.3 Modifying yeast through strategies such as gene knockout.3, 5
Fig.3 Modifying yeast through strategies such as gene knockout.3, 5
 Fig.4 Impact of gene editing on overall trends in GLA N-glycosylation.4, 5
Fig.4 Impact of gene editing on overall trends in GLA N-glycosylation.4, 5

