Monosaccharide Analysis Services
Creative Biolabs is a leading company that has established a comprehensive and advanced glycosylation analysis platform. Based on the latest technologies, we are capable of offering comprehensive monosaccharides analysis services. Our professional scientists are happy to share our knowledge and passion in this field to facilitate worldwide customers' research and project development.
Monosaccharide Analysis
N-glycans and O-glycans consist of various monosaccharides including fucose (Fuc), mannose (Man), N-acetylglucosamine (GlcNAc), N-acetlygalactosamine (GalNAc), galactose (Gal), glucose (Glc) and sialic acids. The number of monosaccharides can be quantified by monosaccharide composition analysis. Besides, it can also detect changes in glycosylation. Monosaccharide analysis is, therefore, a critical and essential way to determine the composition of complex carbohydrates. Moreover, monosaccharide analysis can also be used as an exploratory technique for identifying various monosaccharide modifications (including sulfation and phosphorylation).
Monosaccharide Analysis Service at Creative Biolabs
Creative Biolabs has experienced analysis teams, and we provide clients with comprehensive and high-quality monosaccharide analysis services and ensure that the analysis results are accurate and reliable. In addition to providing analysis services for common polysaccharides such as glucose, fructose, galactose, and mannose, we also provide analysis for some rare monosaccharides, such as Apiose, Xylulose, and Xylonic Acid analysis services. In addition, based on our glycosylation analysis platform, our scientists have in-depth monosaccharide analysis knowledge and technical practical experience, and we provide custom analysis solutions according to client needs.
Our platform provides a serious of technologies for monosaccharide analysis including but not limited to high-performance anion-exchange chromatography with pulsed amperometric detection(HPAE-PAD), high-performance liquid chromatography (HPLC), mass spectrometry (MS), and capillary electrophoresis (CE).
-
HPAE-PAD is a common technology used for glycoprotein glycosylation analysis. The principle of HPAE-PAD is separating carbohydrates with specific interactions between their carboxyl and hydroxyl groups based on composition, size, linkages, charge, and isomerism. HPAE-PAD is a kind of advanced method which can reduce analyst time, expense, and exposure to hazardous chemicals as it doesn't need sample derivatization. Samples are usually releasing the monosaccharides in the acid hydrolyzed way, and then lyophilized, dissolved in water, finally analyzed by HPAE-PAD. Creative Biolabs has accumulated extensive experience in applying HPAE-PAD for monosaccharide analysis. We are happy to offer high-quality custom services by adjusting protocols to meet even the most specific requirements in monosaccharide analysis.
-
HPLC combined with the fluorescent labeling method can stably and adequately determine monosaccharides. Generally, glycoprotein will release monosaccharides in a mild acid hydrolysis procedure. Next, 2-aminobenzoic acid (2AA) is usually used to label the released monosaccharides. At this time, these released monosaccharides are fluorescent, which can be relative quantitatively analyzed by HPLC. Creative Biolabs is proficient at employing HPLC for carbohydrate analysis, and thus you can trustingly hand over any problem to us.
-
MS, in particular liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) and matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), has evolved as one of the most powerful tools for glycan analysis. Creative Biolabs has long been committed to MS technology and confident to provide the most comprehensive services to our clients.
-
CE
CE is suitable for both derivatized and underivatized carbohydrates and it is established as a powerful and versatile separation tool because of the brief analysis time, the ability to handle very complex glycan mixtures with high sensitivity, the minute quantities of samples and reagent required, the capacity to resolve isomeric glycans, and the possibility to acquire high resolution obtained under physiological conditions. Creative Biolabs owns a team of technical experts to deal with any problem you may meet in CE.
Advantages of Our Services
-
Experienced Ph.D. level scientists
-
Comprehensive technology platform
-
High sensitivity and high analysis efficiency
-
Quality after-sale service
Creative Biolabs is a leading company that has developed a panel of technologies to offer the best monosaccharides analysis service. Our platform provides various options, from which you can always find the best match for your particular research. Please feel free to contact us for more information.
Published data
Simple carbohydrates (i.e., monosaccharides) and complex carbohydrates are the two main forms of carbohydrates. Currently, chromatography is regarded as the preferred method for separating and determining the content of monosaccharides because of their high solubility in water. However, the quantitative analysis of different types of monosaccharides is challenging due to the similarity of their structural and chemical properties, as well as the complexity and heterogeneity of the sample matrix. In this study, the authors optimized the extraction and purification steps of the analytes by solid phase extraction (SPE) technology and two ion exchange resins to recover as many monosaccharides as possible from the matrix and effectively remove interfering substances. Subsequently, the authors subjected the purified extracts to derivatization treatments by thiolation and silylation and analyzed each derivative by gas chromatography-mass spectrometry (GC-MS) to determine the recovery rate of the purification step. The results showed that after the analytes were extracted and purified using SPE technology, the monosaccharides and uronic acids in the analytes showed higher recovery efficiency compared with the effect after purification by two ion exchange resins. Among them, the recovery rates of arabinose, xylose, glucose, and glucuronic acid were all over 70%, and the recovery rates of the remaining analytes were over 50%.
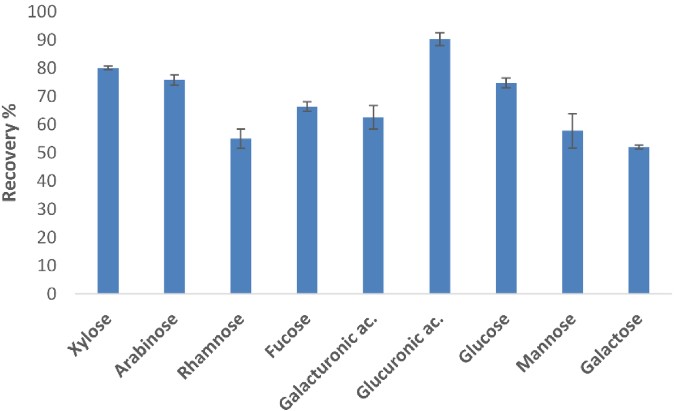 Fig.1 Recovery of analytes after SPE purification.1
Fig.1 Recovery of analytes after SPE purification.1
FAQs
Q1: What specific monosaccharides can be analyzed with your HPAE-PAD method?
A1: Our HPAE-PAD method analyzes a wide range of monosaccharides, including Fuc, Man, GlcNAc, GalNAc, Gal, Glc, etc. Due to its high resolution and sensitivity, it can accurately quantify these sugars without the need for sample derivatization.
Q2: Can you explain the advantages of using MS for monosaccharide analysis over other methods?
A2: MS, especially LC-ESI-MS and MALDI-TOF MS, offers several advantages over other methods, such as high sensitivity, high precision, and the ability to analyze complex glycan structures. It directly identifies and quantifies monosaccharides, even in heterogeneous mixtures. MS also detects monosaccharide modifications such as sulfation and phosphorylation, providing a comprehensive analysis not easily achieved with other methods.
Q3: Can you customize your monosaccharide analysis protocol to meet specific research needs?
A3: Of course. Creative Biolabs prides itself on providing customizable services to meet specific research needs. Our scientists adjust hydrolysis conditions and tailor appropriate detection methods to achieve reliable monosaccharide analysis according to the unique properties of your sample and research goals. We work closely with our clients to ensure that the analysis meets their specific requirements and provides the data needed to advance your project.
Customer Review
Customized Monosaccharide Analysis Service
"Creative Biolabs tailored the monosaccharide analysis protocol specifically for the unique requirements of our project. They adjusted the HPAE-PAD technology to meet our specific needs, ensuring data accuracy and reliability, which was critical for our biomarker discovery."
Comprehensive Analysis Platforms Facilitate Clients' Decision-making Process
"Creative Biolabs' broad technology platform enabled us to select the most appropriate method for a specific study. Whether it was HPAE-PAD, HPLC, MS, or CE, their extensive expertise ensured high-quality monosaccharide analysis results and facilitates our decision-making process."
Reference
-
Bargagli, Irene, et al. "Testing Clean-Up Methods for the Quantification of Monosaccharides and Uronic Acids." Applied Sciences 12.24 (2022): 12781. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 Recovery of analytes after SPE purification.1
Fig.1 Recovery of analytes after SPE purification.1

