O-Linked Glycoengineering Service in Mammalian Cell
O-Glycosylation in Mammalian Cells
O-glycosylation takes place within the Golgi apparatus without a well-defined consensus sequence. Mucin-type glycosylation is the most prevalent form of O-linked glycosylation. O-linked mucin structures exhibit greater compactness and diversity compared to N-linked structures. During O-glycosylation, individual monosaccharides are added to specific serine or threonine residues on proteins by site-specific glycosyltransferases. These core O-glycan structures can undergo further modification or extension through the addition of sugars like galactose, GlcNAc, and sialic acid. This process results in the formation of extended linear or branched structures, which are commonly observed in mammals and frequently conclude with sialic acid.
In CHO cells, the O-glycan repertoire is primarily characterized by simple and di-sialylated core 1 O-glycans and it exhibits a limited monosaccharide diversity. CHO cells lack the glycosyltransferase activity to elongate or branch core 1 O-glycans, and can't produce core 3 O-glycans, which are synthesized by C3 3GnT6. Glycoproteins derived from CHO cell lines typically may lack complex terminal modifications. Although CHO cells are used for producing many O-glycoprotein therapeutics, some O-glycoproteins can't be efficiently produced in CHO cell lines due to these limitations.
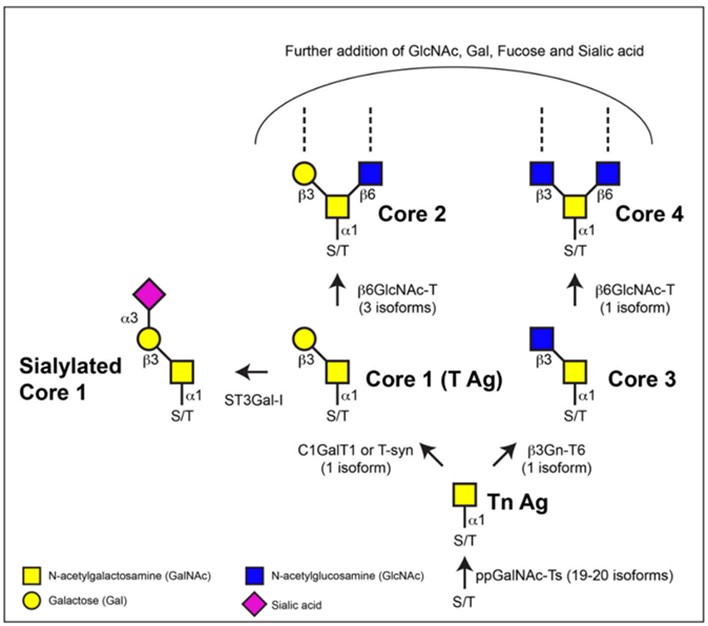 Fig.1 Biosynthesis of mucin-type O-glycans.1, 2
Fig.1 Biosynthesis of mucin-type O-glycans.1, 2
O-Linked Glycoengineering Services in Mammalian Cell at Creative Biolabs
While research on O-linked glycoengineering is relatively limited, it holds significant biological relevance. O-glycans play a crucial role in various biotherapeutic applications, influencing factors such as pharmacokinetics and the binding affinity of peptide-antibody fusions. At Creative Biolabs, a leading provider of comprehensive Glycoengineering Services, O-linked glycosylation can be deliberately modified through genetic engineering techniques in mammalian cells, notably CHO cells. This modification is achieved primarily by introducing specific glycosyltransferases to control glycan elongation and branching, which proves invaluable for advancing bioprocess development toward producing mucin-type recombinant proteins.
For instance, Transient Overexpression, involving extended C1 3GnT3, C2 6GnT1, and C3 3GnT6, may generate extended core 1 and core 3 O-glycans, while increasing the expression of core 2 O-glycans. These structures can be further modified through ST6Gal-I with the addition of terminal 2,3-sialylation. Following the expression of ST6Gal-I, O-glycans featuring terminal 2,6-linked sialylation can be produced.
O-Glycoprotein Therapeutics Produced in CHO Cells
Many biopharmaceuticals typically feature one to several O-glycan structures. Examples of CHO-derived biopharmaceuticals that feature O-linked glycans include rhEPO, human chorionic gonadotropin, human Factor VIII, and Factor IX. Additionally, there are several Fc-fusion protein biopharmaceuticals have been deliberately engineered to incorporate O-glycan structures.
Advantages of Our Services
-
Transient gene overexpression with high-level expression vectors
-
Custom-designed O-glycans through the introduction of specific GTs
-
High-yield production of recombinant O-glycoproteins
-
Professional team with years of experience in glycoengineering
Creative Biolabs takes pride in offering O-linked glycoengineering services in mammalian cells through genetic engineering, enabling the production of diverse O-glycoprotein therapeutics. If you have any specific inquiries about your glycoengineering requirements, please don't hesitate to contact us for further information.
References
-
Tran, Duy T., and Kelly G. Ten Hagen. "Mucin-type O-glycosylation during development." Journal of Biological Chemistry 288.10 (2013): 6921-6929.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 Biosynthesis of mucin-type O-glycans.1, 2
Fig.1 Biosynthesis of mucin-type O-glycans.1, 2

