Plasma Glycoprofiling Service
Advancing Disease Detection: Professional Plasma Glycoprofiling Service at Creative Biolabs
Plasma proteins are mostly N- or O-glycosylated. Glycosylation changes are closely related to different diseases, and multiple studies have shown that changes in glycosylation are observed in the glycocalyx of cancer cells. This discovery has prompted scientists to focus on studying new biomarkers according to glycosylation changes in biological samples for early detection of cancer. Advanced glycan analysis technologies are applied to the detection of various biological samples. In recent years, with the rapid development of analytical technology and instruments, especially the progress in mass spectrometry (MS), glycan analysis of various sources has become reliable and efficient. Creative Biolabs has first-class glycan analysis technology and provides diversified biological sample analysis services to clients around the world. For example: Serum, plasma, Urine, and other biological samples.
We provide professional plasma glycoprofiling service, which usually requires a series of steps to be performed on the tested plasma samples.
Sample pretreatment
First, solid particles and cell residues in plasma samples are removed by centrifugation.
Removal of protein
The plasma sample is then mixed with ammonium bicarbonate and sugar alcohol aqueous solution, glycosidase is added to promote the release of glycans, and the mixture is incubated at 37°C. Proteins are then removed from the glycans using ethanol precipitation, ultrafiltration, or other protein removal techniques to allow for better analysis of the glycans.
Enrichment of glycans
We centrifuge the mixture, take the supernatant and dry it under vacuum, then reconstitute the dried sample with pure water, and use affinity chromatography, gel filtration, ion exchange chromatography, and other methods to analyze the glycans in plasma. Purification and enrichment are performed to improve detection sensitivity and specificity.
Hydrolysis or enzymatic hydrolysis
Based on actual detection needs, we will perform enzymatic or hydrolysis on the enriched glycan sample to obtain smaller monosaccharide units or specific glycan fragments.
Glycan analysis
The glycan analysis in plasma is performed using a variety of technologies, including the following:
-
Chromatography: such as gel permeation chromatography (GPC) and high-performance liquid chromatography (HPLC), which can perform qualitative and quantitative analysis of glycans.
-
Mass spectrometry technologies: such as time-of-flight mass spectrometry (TOF-MS) can be used for structural identification and analysis of glycans.
-
Nuclear magnetic resonance (NMR): It provides structural information about glycans, including bonding methods, conformations, and intermolecular interactions.
-
Glycomic technology: It is used to study and analyze carbohydrate compounds in biological samples and can help identify and characterize glycans in plasma.
-
Fluorescent labeling technology: Fluorescent labeling agents are used to label glycans, and then the glycans are detected and analyzed through fluorescence spectroscopy.
These techniques are applied individually or in combination to provide an in-depth analysis of the composition, structure, and functions of glycans in plasma, providing important information for research.
Published data
Studies have shown that the complement system and C3 glycoprotein play a key role in type 1 diabetes (T1D) disease. In this study, the authors proposed a new and efficient liquid chromatography-mass spectrometry (LC-MS) analysis method for glycoproteomics, aiming to perform high-throughput, site-specific N-glycosylation analysis of C3 glycoprotein and evaluate its potential application in T1D disease. The authors analyzed the N-glycoprofiling in plasma samples from patients with T1D and their healthy siblings. The results showed that the high-mannose sugar was significantly changed in the plasma of patients with T1D, and these glycosylation changes might be closely related to the presence of endoplasmic reticulum stress, which might mark the development of the disease and the progression of T1D itself. Therefore, the analysis of N-glycoprofiling can be used as an important diagnostic tool for monitoring T1D disease in the future.
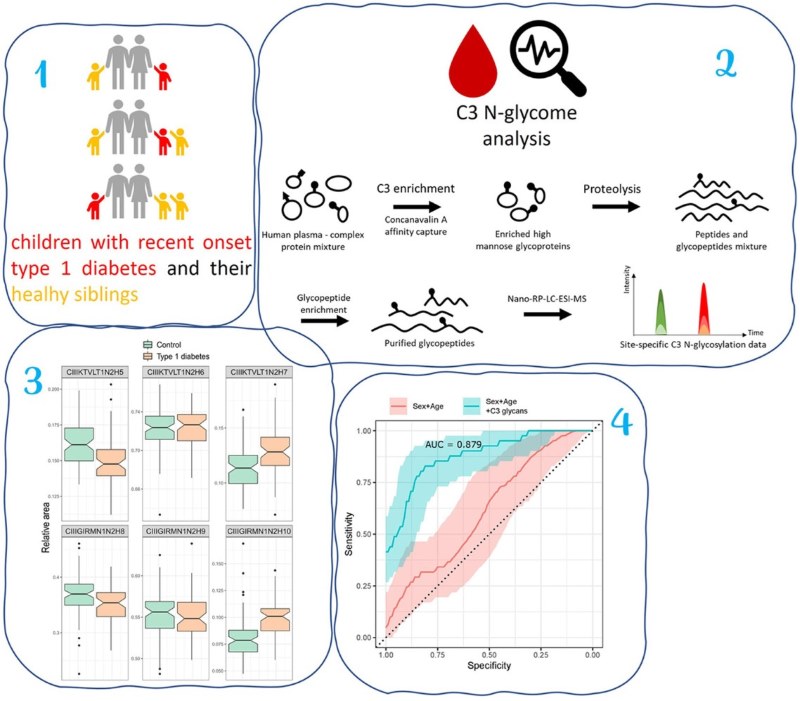 Fig.1 Plasma N-glycan analysis in patients with T1D.1
Fig.1 Plasma N-glycan analysis in patients with T1D.1
Advantages
-
Comprehensive service: We provide a full range of plasma glycoprofiling services from sample collection to data interpretation to meet the various needs of clients.
-
Advanced technology: We provide advanced bio-spectroscopy technologies, including mass spectrometry and nuclear magnetic resonance, for precise data collection and analysis to support plasma glycoprofiling.
-
Personalized solutions: We custom plasma glycoprofiling solutions according to client needs and flexibly respond to different research purposes and needs.
Application
-
Research on disease mechanisms: Plasma glycoprofiling provides an in-depth understanding of the pathogenesis and physiological processes of the disease, and provides important clues for research in related fields.
-
Drug development: Plasma glycoprofiling is used to evaluate the safety and effectiveness of drug candidates and provides support for drug clinical trials.
-
Cancer monitoring: Plasma glycoprofiling is also used for early diagnosis of cancer, monitoring of treatment effects, and assessment of prognosis. By detecting metabolites in plasma, the metabolic characteristics of cancer cells are revealed, providing an important reference for cancer diagnosis and treatment.
Creative Biolabs continues to track the development of Glycoprotein Analysis technology, continuously improves analysis methods and workflows, and always maintains a leading position in technology. Creative Biolabs is committed to offering clients comprehensive and efficient plasma glycoprofiling services. Please feel free to contact us if you need high-quality glycoprofiling, we are your loyal and reliable partner.
FAQ
Q1: Why do we need to perform glycoprofiling in plasma?
A1: Glycoprofiling in plasma is an important means of studying the relationship between glycosylation changes and diseases because glycosylation modifications reflect cells' physiological and pathological states. Many studies have shown that diseases such as cancers cause significant changes in glycosylation patterns in plasma, and these changes serve as potential biomarkers for early detection, monitoring treatment effects, and evaluating prognosis. Therefore, glycoprofiling provides important information support for the in-depth understanding of disease mechanisms and the development of new diagnostic methods.
Q2: How to ensure the accuracy and reliability of glycoprofiling results?
A2: We use advanced analytical techniques such as MS and NMR, combined with a variety of chromatographic methods for comprehensive analysis. Moreover, we strictly follow the laboratory standard operating procedures and implement detailed quality control measures to ensure that each step is accurately calibrated and verified, providing clients with high-quality and reliable analysis results.
Q3: Can your analysis process be customized?
A3: Yes, we customize the analysis process according to the specific needs of our clients. This includes selecting appropriate sample processing methods and analysis techniques to meet different research purposes.
Customer Review
Analysis Accuracy
“Creative Biolabs' glycoprofiling results are extremely accurate and consistent with our previous research data, which greatly enhances our confidence. Through glycoprofiling, we have gained new insights into the relationship between glycosylation changes and disease mechanisms, which will promote our related research.”
Customized Glycoprofiling
“Creative Biolabs has customized glycoprofiling according to our specific needs, allowing us to focus on key biomarkers and improve research efficiency.”
Reference
-
Šoić, Dinko, et al. "High-throughput human complement C3 n-glycoprofiling identifies markers of early onset type 1 diabetes mellitus in children." Molecular & Cellular Proteomics 21.10 (2022). Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services


 Fig.1 Plasma N-glycan analysis in patients with T1D.1
Fig.1 Plasma N-glycan analysis in patients with T1D.1

