Strategies of Genetic Glycoengineering
Glycoengineering is increasingly recognized as an important strategy in the development of new biopharmaceuticals with optimized half-life, antigenicity, and efficacy. With years of experience and advanced technologies, Creative Biolabs has proposed different genetic strategies to circumvent issues like heterogeneity, nonhuman glycans in different glycoprotein production systems. Professional technical experts in Creative Biolabs are dedicated to providing the most comprehensive glycoengineering service and we offer our clients with high-quality services in your glycoprotein project to help you get milestone success.
Strategies of Genetic Glycoengineering
Glycosylation profile varies significantly across organisms and glycosylation strongly affects therapeutic activity and efficacy of glycoprotein biologics. In general, the process of glycosylation starts in the endoplasmic reticulum (ER). Many glycosylation steps are accomplished by Golgi-localized glycosyltransferases that subsequently generate mature glycan structures. Thereby, numerous genetic strategies have been proposed for manipulating glycosylation altering heterogeneity, sialylation, fucosylation, and branching structures and humanizing the glycosylation profile of expression hosts, such as genes knockdown, knockout and knockin, overexpression and alteration and so forth.
-
Many experimental cases have demonstrated the effectiveness of gene knockout in glycosylation engineering. For example, engineered Chinese hamster ovary (CHO) cells (with GnT-IV and -V overexpression and GalT4 knockdown) express a highly glycosylated human chorionic gonadotropin (hCG) hormone. An RNAi-mediated knockdown approach was used to reduce plant-specific glycosyltransferase activities. Besides, double siRNA knockdown of α1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD) in CHO cells provide a new strategy for generating fully non-fucosylated therapeutic antibodies with enhanced ADCC.
-
In glycoengineering approaches towards optimizing activity, unwanted enzymes are typically inactivated to prevent the transfer of single monosaccharides to glycan structures. Knockout of core α1,6-fucosyltransferase (FUT8) in CHO, knockout of α1,6-mannosyltransferase (Och1p) in yeast, knockout of core α1,3-fucosyltransferase (FUT) and β-N-acetylglucosaminidase (β-hex) in insect cells, knockout of FUT and β1,2-xylosyltransferase (XylT) in plants have already been reported. The synthesis of undesired or host specific glycol-epitopes also could be eliminated by employing gene knockout strategies. Besides, knockin strategies using human-specific glycosyltransferases have emerged for the humanization of N-glycosylation in plants. Glycoengineering with the combined knockout/knockin approach of glycosylation-related enzyme genes has been recognized as an effective method for the introduction of human glycosylation machinery.
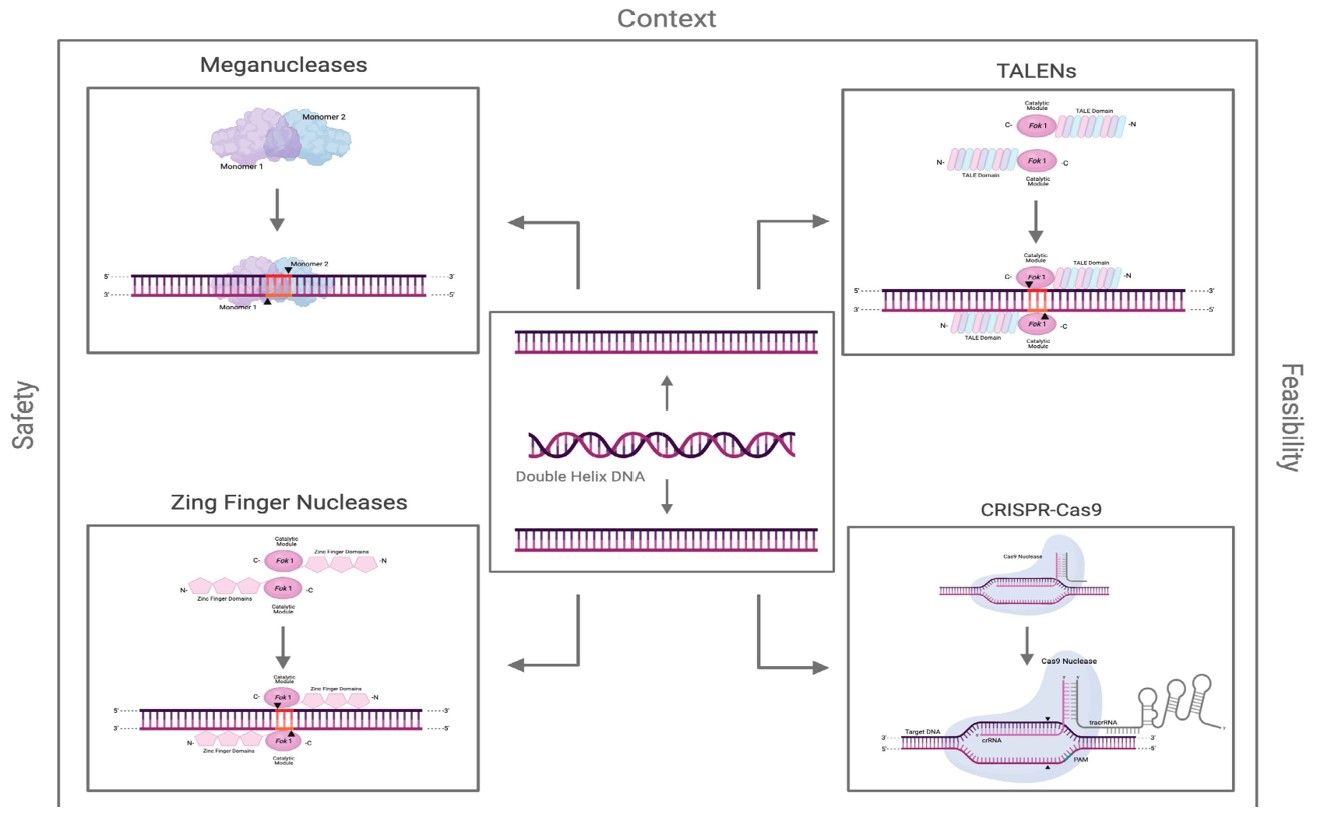 Fig.1 Some genome editing technologies.1, 3
Fig.1 Some genome editing technologies.1, 3
-
Besides knockout and knockin of sialyltransferase genes, increases in sialic acid content can be obtained by overexpressing specific genes, such as galactosyltransferase genes, anti-apoptotic genes, and genes involved in sialic acid biosynthesis and transport. Additionally, co-overexpressing several genes together enhanced sialylation more significantly than overexpressing single genes. The altered glycan ratios can be overcome by overexpressing ST6Gal 1 in CHO cells by using a plasmid expression vector. Enhancement of sialylation on humanized IgG-like bispecific antibody can be obtained by overexpression of α2,6-sialyltransferase derived from CHO cells. Co-overexpression of β1,4-N-Acetylglucoseaminyltransferase III (GnT III) and α-mannosidase II leads to further lower fucose content by introducing non-fucosylated hybrid-type glycans. Production of antibodies with increased antibody effector function was also achieved by overexpression of GnT-III in CHO cells. For sialylation, overexpression of α-2,3-sialyltransferase and β-1,4-galactosyltransferase elevates IgG sialylation and galactosylation.
-
Glycan heterogeneity in recombinant glycoproteins can be categorized into macro-heterogeneity and micro-heterogeneity. Macro-heterogeneity is associated with variations in glycosylation site occupancy. Overexpression of the subunit of the OST, LmSTT3, in Pichia pastoris leads to a significant increase in the N-glycan site occupancy. Extra N-glycosylation sites can be introduced into recombinant human erythropoietin (rhEPO) through modification of glycosylation consensus. Micro-heterogeneity refers to the diversity of glycan structures at the same glycosylation site and can be overcome by genetic editing of glycosyltransferases. Combined knockout of certain N-acetylglucosaminyltransferases (GnT) genes enables the synthesis of rhEPO featuring homogenous bi-antennary N-glycans. The introduction of ST6Gal-I into CHO ST3Gal4/6 knockout cells produces N-glycans characterized by exclusive α-2,6 sialylation.
-
Glycoengineering to manipulate sialylation mainly focused on increasing the α-2,6 sialylation and the sialic acid content, especially for the production of therapeutic glycoproteins. Genetic modulations such as knock-in and overexpression for several sialyltransferase genes have been employed to manipulate sialylation. Introducing α2,6-SiaT in CHO cells has been reported to elevate the sialylation levels of the recombinant IgG. Co-overexpressing β1,4-GalT and α2,6-SiaT in CHO cells has enhanced the α2,6 sialylation of antibodies. Additionally, increased sialic acid content can be obtained by overexpressing CMP sialic acid synthetase and CMP-sialic acid transporter.
-
Glycoengineering is extensively utilized to produce therapeutic glycoproteins with reduced or removed fucosylation, as non-fucosylated antibodies induce higher ADCC. Genetic modifications targeted at different enzymes have been used to control the fucosylation processes. One common approach is to knock out the core α1,6-fucosyltransferase (FUT8) responsible for the synthesis of α1,6 core fucosylated N-glycans. Another strategy involves disrupting the GDP-fucose synthesis pathway or inactivating the GDP-fucose transporter. Additionally, overexpression of β-1,4-N-acetylglucosaminyltransferase III (GnT-III) responsible for adding bisecting GlcNAc, may generate a structure unsuitable for fucosylation.
-
The branching or antennary of N-glycans is controlled by enzymes like GnT-I, GnT-II, GnT-IV, and GnT-V. Manipulation of N-glycan branching can be achieved by genetically regulating these enzymes. Independently overexpressing GnT-IV or GnT-V enzyme in CHO cells enables the enhanced generation of tri-antennary structures. Combined overexpression of GnT-IV and GnT-V enzymes leads to further amplified branching, resulting in significantly elevated tetra-antennary structures.
-
Glycosaminoglycans (GAGs) serve vital roles in biology and have various medical uses. Genetic glycoengineering improves GAG properties and therapeutic potential. For example, overexpressing NDST2 and 3OST1 in CHO cells enhances GAG secretion. Introducing HS2ST1 increases the synthesis of 2-O-sulfated disaccharides. Co-expressing HS6ST1 and NDST2 generates N-,6-O-disulfate disaccharides. To expand chondroitin sulfate biosynthesis, CHST3, UST, and CHST15 induce 6-O-sulfation, 2,4-O-sulfation, and 4,6-O-disulfation, respectively.
Features of Genetic Glycoengineering Strategies
-
Optional genetic approaches for manipulating glycosylation
-
Different production system
-
Perfect after-sale service system
-
Cost-effective and time-saving
Manipulating glycosylation to increase desired glycans in glycoprotein biologics is of high scientific and commercial interest. Equipped with state-of-the-art research and manufacturing facilities, Creative Biolabs has gained significant knowledge and rich experience in genetic glycoengineering. We are more than happy to share our experience and help our customers in cell line glycoengineering development. Please contact us for more information and a detailed quote.
Published data
Efficient production of glycoproteins can be effectively achieved through cell line genetic glycoengineering. Various strategies have been developed for glycoengineering. This paper introduces us to some genetic glycoengineering strategies for CHO cells, including knockdown, knockout, overexpression, etc. The availability of genomic sequences allows CHO glycoengineering to utilize a variety of engineered nucleases, including clustered regularly interspaced short palindromic repeats (CRISPR) systems, transcription activator-like nucleases (TALENs), etc., which have a high degree of precision. CRISPR/CRISPR associated protein 9 (CRISPR/Cas9) is the main knockdown and overexpression of genes in CHO cells strategy. These genetic glycoengineering strategies summarized in this paper provide theoretical support for us to better design and develop cell line glycoengineering.
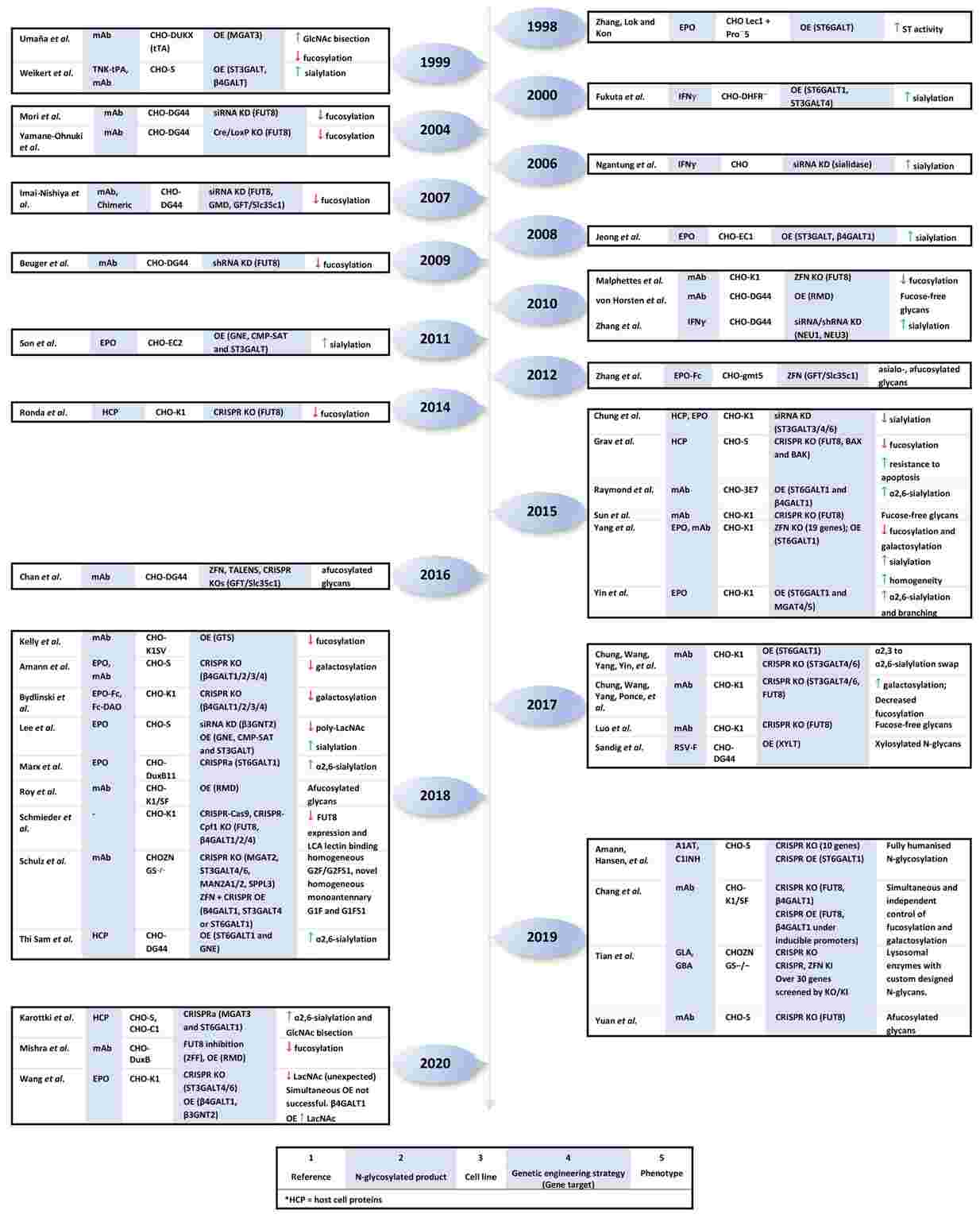 Fig.2 Genetic engineering in CHO cells.2, 3
Fig.2 Genetic engineering in CHO cells.2, 3
FAQs
Q1: What gene editing techniques have been developed for genetic glycoengineering?
A1: Several gene editing technologies have been developed for genetic glycoengineering, such as meganucleases, zinc finger nucleases, TALENs, CRISPR/Cas9, etc. These technologies open up the possibility of realizing gene insertion, knockout, and so on. It is important to consider the impact of various aspects when selecting a gene editing technology.
Q2: What are the applications of genetic glycoengineering?
A2: Genetic glycoengineering provides an efficient route for research in the field of glycobiology and biomedicine. It has a wide range of applications in glycosylation pathway research, glycosylation functions research, therapeutic glycoprotein production, cell system and animal model development, and vaccine development.
Customer Review
Comprehensive Insights into Genetic Glycoengineering
"We appreciated the comprehensive insights into genetic glycoengineering provided by the experts at Creative Biolabs. They helped us precisely and purposefully customize glycan structures and regulate glycosylation pathways. The team's extensive knowledge, coupled with advanced technology, accelerated our research time and optimized our antibody products. It was a great experience for us."
Unrivaled Solutions for Genetic Glycoengineering
"We were impressed by the deep knowledge of glycosylation pathways and genetic glycoengineering at Creative Biolabs. From initial glycosylation design to cell line modification, their team developed unparalleled solutions and the results were outstanding. Thank them for their excellent service and expertise in glycoengineering. We look forward to future collaborations."
References
-
González Castro, Nicolás, et al. "Comparison of the feasibility, efficiency, and safety of genome editing technologies." International Journal of Molecular Sciences 22.19 (2021): 10355.
-
Donini, Roberto, Stuart M. Haslam, and Cleo Kontoravdi. "Glycoengineering Chinese hamster ovary cells: a short history." Biochemical Society Transactions 49.2 (2021): 915-931.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 Some genome editing technologies.1, 3
Fig.1 Some genome editing technologies.1, 3
 Fig.2 Genetic engineering in CHO cells.2, 3
Fig.2 Genetic engineering in CHO cells.2, 3

