Thin Layer Chromatography (TLC) for Carbohydrate Analysis
Thin-layer chromatography (TLC) is a planar chromatographic technique extensively used as a relatively rapid and straightforward analytical technique for glycan analysis based on their relative hydrophobicity. Creative Biolabs has explored an efficient strategy for the analysis of not only neutral monosaccharide and oligosaccharide, but also for acidic monosaccharides, disaccharides, and oligosaccharides. We are more than happy to share our experience to help our customers in carbohydrate analysis.
TLC for Carbohydrates Analysis
TLC is a method for separating compounds by their rate of movement through a thin layer of silica gel coated on a glass plate. It is one of the easiest and most versatile methods due to its low cost, simplicity, quick development time, high sensitivity, and good reproducibility. TLC is routinely used by synthetic organic molecules and natural products working on a variety of types of molecules. One class of organic natural products that have historically seen less use of TLC is carbohydrates. Carbohydrates are highly polar molecules and often require derivatization to be analyzed by TLC. This is primarily because their polar nature makes it difficult to separate these molecules on commonly used polar supports, such as silica and alumina, and also complicates the selection of eluents. Before the advent of modern chromatographic methods, carbohydrates in the past largely relied on paper chromatography for rapid and inexpensive analysis. Nowadays, TLC has been paid more and more attention due to its rapidity, cost-efficient optimization of separation (mobile and stationary phases can be changed easily), the high sample throughput in a short time, and the suitability for carbohydrate analysis.
TLC for Carbohydrates Analysis Service at Creative Biolabs
The in-depth characterization of glycan structures is crucial to understand their structure-function relationships and their effects on health and various diseases. Despite advances in rapid analysis, the utility of mass spectrometry (MS) is limited for complex mixtures of carbohydrates due to their low ionization efficiency and the difficulty in separating oligosaccharides because of their high structural similarity. Base on TLC technology, Creative Biolabs has developed a skillful strategy for simultaneous and rapid separation, detection, and identification of carbohydrates to achieve efficient carbohydrate profiling. Our strategy is not only suitable for the analysis of neutral monosaccharide and oligosaccharide, but also for acidic monosaccharides, disaccharides, and oligosaccharides, which pose an even more difficult challenge as they contain a formal negative charge and counterion, and can interact with commonly used stationary phases.
Samples Available Include but Not Limited To:
-
Plant- or animal-derived carbohydrates
-
Neutral monosaccharide, oligosaccharide and polysaccharide
-
Acidic monosaccharide, oligosaccharide and polysaccharide
-
Glycolipid
-
Ganglioside
Experienced in applying TLC for carbohydrates analysis, Creative Biolabs is fully competent and dedicated to serving as your one-stop-shop for carbohydrates analysis. For more detailed information, please feel free to contact us or directly send us an inquiry.
Published data
Honey is a highly concentrated sugar solution, mainly containing glucose and fructose, as well as small amounts of disaccharides, trisaccharides, and oligosaccharides. It can be used as a nutritious food with potential medicinal value and biological activity. However, there is a phenomenon of honey adulteration in the market, which has a serious negative impact on the honey industry. Common adulterants include industrial-grade sugars such as sucrose syrup, maltose syrup, and corn syrup. Therefore, in response to this market phenomenon, it is necessary to develop analysis methods to detect the authenticity of honey. Due to the lack of chromophores in sugars, Ultraviolet detection methods are not ideal. Therefore, in this study, the authors developed a method combining high-performance thin-layer chromatography (HPTLC) and organic extract spectrum for the detection and identification of adulterants in honey, and the detection method was validated. The results showed that the method combining the HPTLC sugar spectrum and organic extract spectrum could easily detect sugar adulteration. Fructose and glucose were the main components of honey, and their contents could be used to identify the authenticity of honey. Moreover, combined with HPTLC fingerprinting of organic extracts, the contents of sucrose and maltose could be used to determine the type of adulterants. In conclusion, the HPTLC analysis method developed by the authors was simple, cost-effective, and could accurately detect adulteration in honey and identify the types of adulterants, which will help to conduct reasonable quality control in the honey industry.
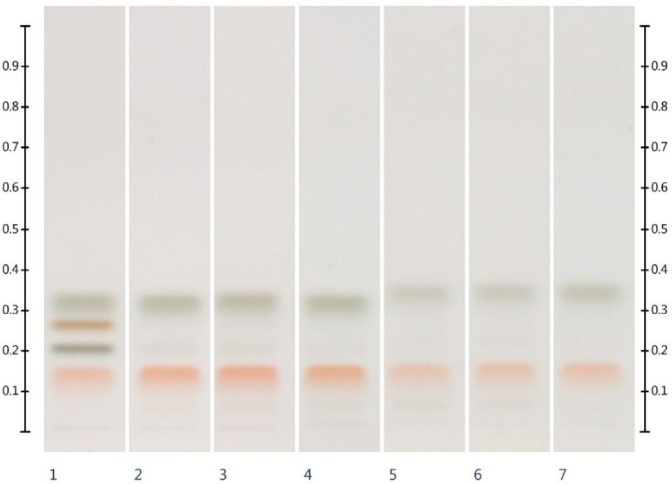 Fig.1 HPTLC sugar spectrum analysis results of various sugar standards and six types of honeys.1
Fig.1 HPTLC sugar spectrum analysis results of various sugar standards and six types of honeys.1
FAQs
Q1: What types of carbohydrates can you analyze using TLC?
A1: We have developed methods to analyze a wide range of carbohydrates using TLC, including:
-
Neutral monosaccharides and oligosaccharides: Such as glucose, fructose, and maltose.
-
Acidic monosaccharides, disaccharides, and oligosaccharides: Such as glucuronic acid and N-acetylneuraminic acid.
-
Polysaccharides: Naturally occurring polymers such as starch and cellulose.
-
Glycolipids and gangliosides: Complex molecules composed of polysaccharide chains attached to a lipid moiety.
Our advanced separation techniques can effectively distinguish between these different types of carbohydrates.
Q2: How do you separate carbohydrates with very similar structures?
A2: For carbohydrates with very similar structures, we use the following methods to separate carbohydrates.
-
High-resolution TLC: Use high-resolution TLC plates to achieve better separations.
-
Developing multiple separation systems: Apply different solvent systems to maximize separation specificity.
-
Derivatization: Derivatization enhances the detection and separation of carbohydrates by making them less polar and more hydrophobic.
Q3: What are the main advantages of using TLC for carbohydrate analysis compared to other chromatographic techniques?
A3: TLC is a cost-effective, simple, and rapid method compared to more complex methods such as HPLC or MS. It allows easy adjustment of the mobile and stationary phases, and can process multiple samples simultaneously with high sensitivity and reproducibility.
Customer Review
Highly Sensitive And Reproducible Carbohydrate Analysis
"We utilized Creative Biolabs' TLC service to analyze neutral and acidic carbohydrates in plant extracts. The sensitivity and reproducibility of their technology were excellent. Oligosaccharides could be detected at minute levels, which was critical for our project. The results were consistent across multiple runs, providing reliable data for our research."
High-throughput Analysis Service
"We needed to analyze a large number of samples in a short period, and Creative Biolabs' TLC service was ideal for this purpose. The high-throughput capabilities allowed them to quickly process all samples, always maintaining a high analytical standard. This efficiency had helped facilitate our project schedule."
Reference
-
Islam, Md Khairul, et al. "Sugar profiling of honeys for authentication and detection of adulterants using high-performance thin layer chromatography." Molecules 25.22 (2020): 5289. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 HPTLC sugar spectrum analysis results of various sugar standards and six types of honeys.1
Fig.1 HPTLC sugar spectrum analysis results of various sugar standards and six types of honeys.1

