Next-IO™ A2AR Therapeutic Monoclonal Antibody Program
About This Program
This program aims to develop A2AR therapeutic monoclonal antibody for non-small cell lung cancer immunotherapy.
Rationale for our program:
-
Advanced non-small cell lung cancer (NSCLC) cannot be cured, with a median survival of 12 months. There is an urgent need for more effective treatments.
-
Immunotherapy has recently been found to produce a clinical response in NSCLC with a significant improvement in median OS.
-
The adenosine A2A receptor is an immunological checkpoint protein that is frequently expressed on human lung cancer TIL and produces immunosuppression within the tumor.
These data support the rationale for the development of the first-in-class human A2AR Abs for NSCLC.
A2AR
The adenosine A2A receptor (A2AR) belongs to the G protein-coupled receptor (GPCR) family A or rhodopsin receptor family. Adenosine receptors include four subtypes (A1R, A2AR, A2BR, and A3R) that are activated by extracellular adenosine. A2AR and A1R are widely distributed throughout the central nervous system and surrounding, while low-density A2BR and A3R are present in the brain.
Research to date strongly supports the A2a receptor as a new way of immunotherapy:
-
The tumor microenvironment has a relatively high concentration of adenosine. Several studies suggest that ubiquitous nucleoside adenosines that act through the A1, A2A, A2B and A3 receptor (AR) subtypes play a crucial role in tumor development.
-
Blocking A2a receptor activation has the potential to significantly enhance anti-tumor immunity in a mouse model.
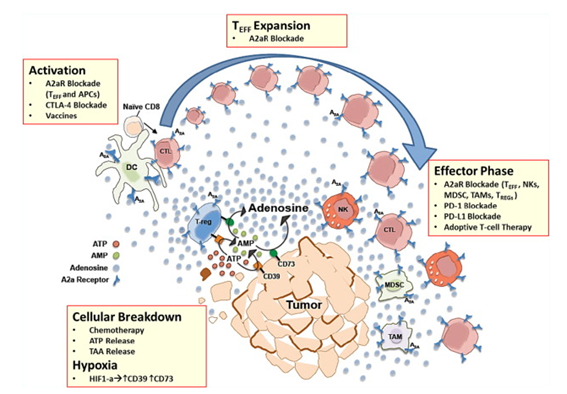 Fig.1 A2aR blockade in the tumor microenvironment.1,3
Fig.1 A2aR blockade in the tumor microenvironment.1,3
Supporting Data
-
NSCLC patients with high A2AR expression have significantly better overall survival (left) and recurrence-free survival (right).
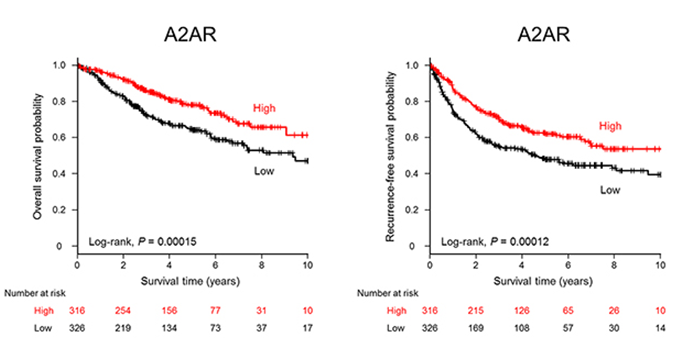 Fig.2 A2AR expression levels in NSCLC patients .2,3
Fig.2 A2AR expression levels in NSCLC patients .2,3
NSCLC
-
The global NSCLC drug market is expected to post a CAGR of more than 13% during the period 2019-2023.
-
The global NSCLC drug market is expected to increase by more than $25 billion between 2018 and 2026.
-
The global non-small cell lung cancer therapeutics market share, by therapy.
Ongoing Clinical Trials
-
Currently, only two anti-A2AR antagonists are being evaluated in clinical Phase I/II stage.
-
We believe that this novel targeting strategy will provide insights into the tumor immunotherapy, especially in the treatment of NSCLC cancer. In an effort to optimally leverage the A2AR-mediated immune response, our next-IO™ A2AR targeted antibody program attempts to explore the optimal combination therapy trials by involving other immunomodulatory agents.
Program Mangement
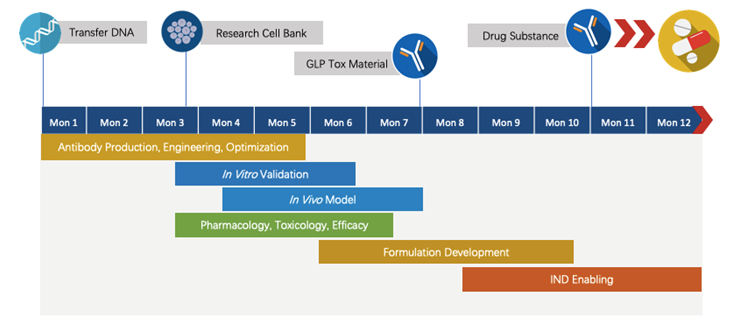
We have extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop A2AR therapeutic monoclonal antibody program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership together. For any partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners further their programs with more chances to succeed. Look forward to cooperating with you in the near future.
References
-
Leone, Robert D., Ying-Chun Lo, and Jonathan D. Powell. "A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy." Computational and structural biotechnology journal 13 (2015): 265-272.
-
Inoue, Yusuke, et al. "Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer." Oncotarget 8.5 (2017): 8738.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use


 Fig.1 A2aR blockade in the tumor microenvironment.1,3
Fig.1 A2aR blockade in the tumor microenvironment.1,3
 Fig.2 A2AR expression levels in NSCLC patients .2,3
Fig.2 A2AR expression levels in NSCLC patients .2,3

 Download our brochure
Download our brochure