ADC Analytical Characterization and Validation
 Empowered by advanced analytical equipment and experienced technicians, Creative Biolabs offers comprehensive end-to-end antibody-drug conjugation (ADC) services including ADC antibody screening, conjugation chemistry, linker and payload service, analytical characterization and validation, fill and finish and other antibody relevant services to help our clients get a promising ADC candidate for drug development. We are fully competent and dedicated to being your reliable companion for comprehensive ADC analytical characterization and validation service.
Empowered by advanced analytical equipment and experienced technicians, Creative Biolabs offers comprehensive end-to-end antibody-drug conjugation (ADC) services including ADC antibody screening, conjugation chemistry, linker and payload service, analytical characterization and validation, fill and finish and other antibody relevant services to help our clients get a promising ADC candidate for drug development. We are fully competent and dedicated to being your reliable companion for comprehensive ADC analytical characterization and validation service.
The assembling of an antibody-drug conjugate (ADC) is the covalent coupling of a critical monoclonal antibody and a druggable payload through a specific linker molecule. The final goal of ADC discovery and development is to deploy the ADC candidate into animal models and eventually patients, in order to treat cancer utilizing the payload efficacy of the ADC. Before deploying the ADC candidate into in vivo systems, in vitro analytical characterization and validation is necessary to capture the basic biochemical characteristics and in vitro efficacy data to evaluate the ADC project. Creative Biolabs provides a series of ADC in vitro analytical characterization and validation services to determine the biochemistry and efficacy of the ADC candidate which can provide directions and guideline for project progression.
Analytical Characterization and Validation Services
Creative Biolabs offers a comprehensive set of in vitro analytical characterization and validation services including: (but not limited to)
We offer biochemical analysis to determine the structure, stability and drug conjugation pattern of the assembled ADC.
-
Structural analysis: To evaluate the structure of antibody or antibody fragment to ensure correct folding after payload chemistry conjugation.
-
Stability analysis: To evaluate the physical stability (auto-fragmentation, aggregation, thermal stability, solubility), chemical stability (in vitro minimum payload release at non-desired buffer, antibody reactivity during and after chemistry conjugation) and serum stability (in vitro minimum payload release in serum), etc.
-
Conjugation sites analysis: To offer the analytical results of conjugated payload-linker location sites through mass spectroscopy, as well as the conjugation information about the unreacted payload-linker on the target antibody.
-
Drug antibody ratio (DAR) determination: To determine the payload drug to antibody molar ratio, as well as the distribution pattern of various DAR species via chromatography and mass spectroscopy analysis.
We offer in vitro efficacy studies to evaluate the cytotoxicity of constructed ADC utilizing various assays.
-
Affinity evaluation: To determine the antigen and Fc receptor binding affinity via different approaches including ELISA, flow cytometry, surface plasmon resonance (SPR).
-
Internalization assays: To evaluate the ADC internalization (incorporation into cells) upon binding via a series of in vitro efficacy assays such as ELISA, flow cytometry.
-
Cytotoxicity assays: To measure the cell viability in a dosage dependent manner utilizing antigen-expressing cell lines.
-
ADCC and CDC assays: To perform antibody dependent cellular cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) assays to provide more details about the pharmacological effects of the ADC.
To mimic the tumor treatment, we offer 3D in vitro platform to evaluate the behavior and cytotoxicity of an ADC using innovated antigen-expressing 3D cultured cells.
-
ADC targeting profile: To evaluate the targeting efficiency, pattern, as well as reflect target distribution.
-
ADC tumor penetration: To provide a 3D distribution profile of the ADC in tumors.
-
ADC tumor efficacy: To measure the efficacy of an ADC in a whole regime which can accurately mimic the scenarios of tumors.
End-to-end ADC Analytical Characterization and Validation
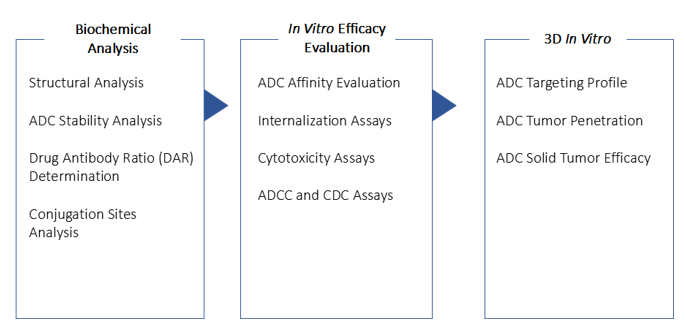 Fig. 1 Our end-to-end ADC analytical characterization and validation services.
Fig. 1 Our end-to-end ADC analytical characterization and validation services.
Highlights
-
High-resolution analytical platforms;
-
Experienced technical personnel;
-
Comprehensive end-to-end ADC in vitro characterization and validation.
ADC has emerged as a promising immunotherapy with high potential in the treatment of cancer. Creative Biolabs offers a series of in vitro assays to provide accurate guidelines for ADC optimization and in vivo testing in next stage. We are dedicated to providing customized solutions to tailor specific service package. Any questions, please feel free to contact us for more information and a detailed quote.
For Research Use Only | Not For Clinical Use



 Fig. 1 Our end-to-end ADC analytical characterization and validation services.
Fig. 1 Our end-to-end ADC analytical characterization and validation services.
 Download our brochure
Download our brochure