About This Program
This program aims to develop anti-4-1BBL therapeutic fusion protein for immuno-oncology.
The 4-1BBL agonistic antibodies, used alone or in combination with other anticancer drugs, have shown various therapeutic efficacies. However, presently, several ongoing clinical trials to evaluate the efficacy of agonistic 4-1BBL antibodies have reported significant hepatotoxicity and other complications in preclinical and clinical studies. In marked contrast, the latest data have dominated that 4-1BB ligand chimeric fusion protein shows robust T-effector responses with therapeutic efficacy in various preclinical tumor models, highlighting a clear rationale for 4-1BBL-FP-based cancer immunotherapy.
4-1BBL
4-1BB ligation on T cells triggers a signaling cascade that results in upregulation of antiapoptotic molecules, cytokine secretion, and enhanced effector function. However, only one known natural 4-1BB ligand (4-1BBL) is expressed as a type II transmembrane protein predominantly on antigen-presenting cells, such as dendritic cells (DC), macrophages and B cells. The membranous form of 4-1BBL exists as a trimer and provides a robust costimulatory signal when it binds to receptors on T cells.
Targeting 4-1BBL for cancer immunotherapy:
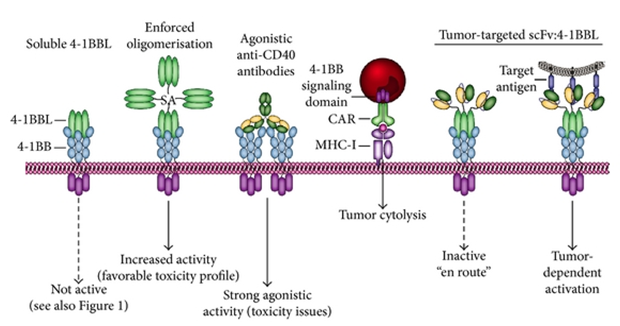 Fig.1 4-1BBL / 4-1BB.1
Fig.1 4-1BBL / 4-1BB.1
-
Soluble 4-1BBL or hexameric 4-1BBL is essentially inactive.
-
Enforced oligomerization using SA-4-1BBL fusion protein enables 4-1BB activation with a favorable toxicity profile.
-
Agonistic 4-1BB antibodies potently activate 4-1BB signaling but with dose-limiting toxicity.
-
The selective use of 4-1BB co-stimulatory signaling can potentiate CAR T-cell activity and trigger effective lysis.
-
Selective delivery of 4-1BBL using scFv: 4-1BBL ensures target antigen-restricted conversion of inactive s4-1BBL into membrane-like and signaling competent 4-1BBL.
4-1BBL Fusion Protein in Cancer Studies
Here are some published data about 4-1BBL fusion protein work as a potential target for cancer immunotherapy.
-
The effect of rh4TFP-2 fusion protein (recombinant human 4-1BBL/Tumstatin fusion protein) therapy on human melanoma tumor.
-
SA-4-1BBL fusion protein is dose-dependent and effective against different tumor types.
Indication
4-1BB:4-1BBL signaling pathway has been shown to have antitumor effects in a number of model systems such as B-cell Lymphoma, melanoma, glioma, and sarcomas. Therefore, we hope to develop multiple fusion protein programs that apply to different indications (not limited to one specific tumor type), of which 4-1BB is highly expressed.
Clinical Trials under Progress and Market Prospect
-
Currently, the 4-1BBL fusion protein is in clinical phase I: FAP-4-1BBL FP for the treatment of solid tumors. (Roche Company)
An increasing number of 4-1BBL fusion proteins are getting confirmed on its role to stimulate T cells and shows great promise in cancer treatment. However, further studies are needed to identify the engineering and conjugation strategies to improve efficacy, safety, and clinical success. Our program will be the pioneer in the field.
-
The global fusion protein market value is expected to grow at a compound annual growth rate between 2019 and 2025. In this case, 4-1BBL fusion protein is a compelling target for novel drug development.
Program Plan
Creative Biolabs has established the novel chimeric fusion protein platform for Agonist Redirected Checkpoint (ARC) program development. We have extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years prior to entering the IND stage.
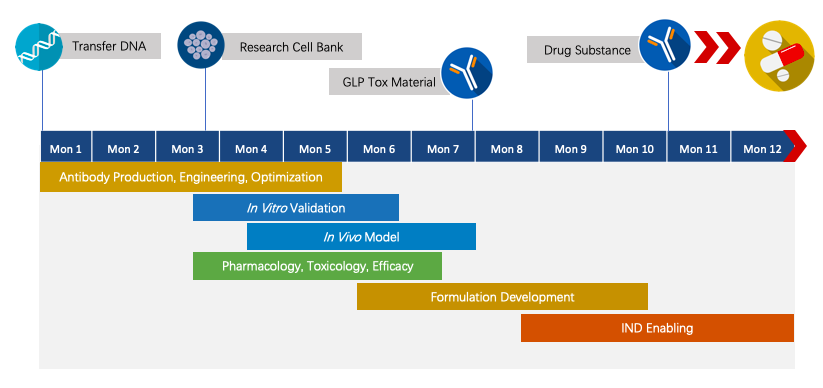 Fig.2 The timeline of Next-IOᵀᴹ programs.
Fig.2 The timeline of Next-IOᵀᴹ programs.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop anti-4-1BBL therapeutic fusion protein program together. Our scientists are dedicated to bringing together years of valuable experience to our partner and achieve a meaningful partnership. By doing so, we wish to help both parties to proceed with IND and many stages of clinical trials beyond.
If you are interested, please feel free to contact us so that we can discuss the program and other possible opportunities for cooperation. Look forward to working with you in the near future.


 Fig.1 4-1BBL / 4-1BB.1
Fig.1 4-1BBL / 4-1BB.1
 Fig.2 The timeline of Next-IOᵀᴹ programs.
Fig.2 The timeline of Next-IOᵀᴹ programs.
 Download our brochure
Download our brochure