About This Program
This program aims to develop anti-CD112 therapeutic monoclonal antibody for immuno-oncology.
CD112 acts as an organizer for Sertoli and sperm cell junctions and synapse formation in testis and neurons. In addition to the cell adhesion function, CD112 mediates the entry and spread of infections from various viruses. It has also been reported that DNAM-1 (CD226) and TIGIT interact with Nectin-2 to activate immune cells. These data highlight a clear rationale for anti-CD112 antibody-based cancer immunotherapy.
CD112
CD112, also known as PVRL2, or nectin-2, is a recently identified surface protein and a member of the PVR-like protein family, which primarily expressed on APCs (such as dendritic cells and macrophages) and tumor cells. CD112R, also known as PVRIG, preferentially expressed on T cells and inhibits T cell receptor-mediated signaling, is a ligand of CD112 with high affinity. CD112R competes with CD226 for binding to CD112. Studies have shown that:
-
Nectin2 plays a prominent role in the establishment of adherens and tight junctions.
-
It acts as a ligand for the activating receptor CD226 and is upregulated on the surface of tumor and virus-infected cells, thereby contributing to cytotoxic lymphocyte-mediated recognition and killing of damaged cells.
-
Disrupting the CD112R-CD112 interaction enhances human T cell response.
-
The inhibitory effect of PVRL2 was mediated by PVRIG but not TIGIT, demonstrating that the PVRIG-PVRL2 pathway is a nonredundant signaling node.
CD112 in Cancer Studies
A newly identified target, only a few studies have investigated the immune‑regulatory effect of CD112 antibody in the tumor microenvironment. Here are some published data about CD112 molecule work as a potential target for cancer immunotherapy.
Clinical Trials under Progress
To our knowledge, there are NO ongoing clinical trials of anti-CD112 therapeutic monoclonal antibody. Our program will be a pioneer in the development of CD112 antibody.
Program Plan
We have extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years prior to entering the IND stage.
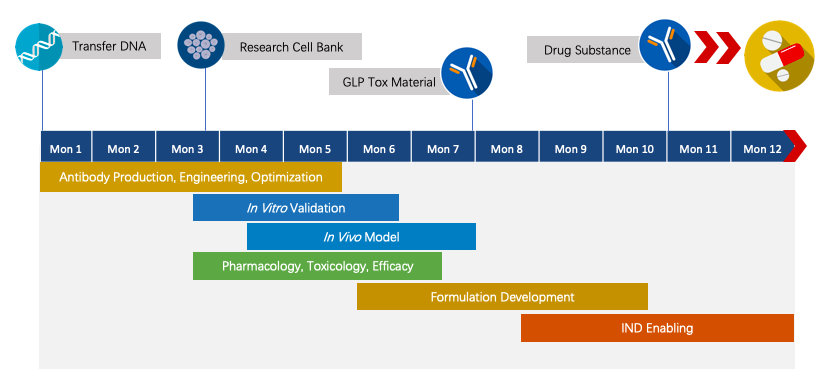 Fig.2 The timeline of Next-IOᵀᴹ programs.
Fig.2 The timeline of Next-IOᵀᴹ programs.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop anti-CD112 therapeutic monoclonal antibody program together. Our scientists are dedicated to bringing together years of valuable experience to our partner and achieve a meaningful partnership. By doing so, we wish to help both parties to proceed with IND and many stages of clinical trials beyond.
If you are interested, please feel free to contact us so that we can discuss the program and other possible opportunities for cooperation. Look forward to working with you in the near future.


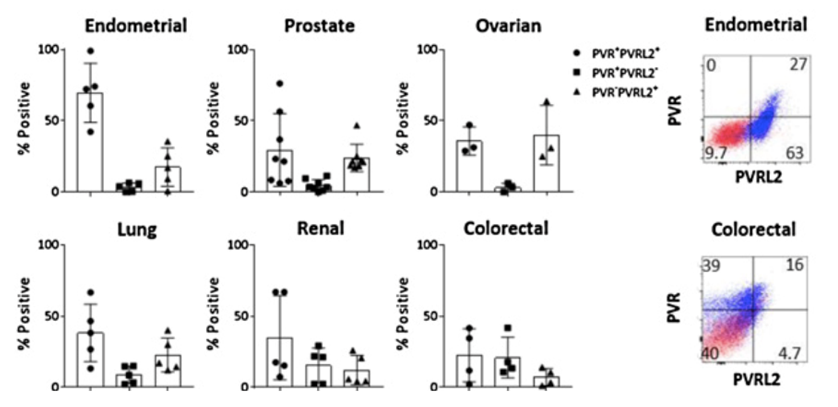 Fig.1 PVRL2 expression is increased in the TME of dissociated tumor samples.1
Fig.1 PVRL2 expression is increased in the TME of dissociated tumor samples.1
 Fig.2 The timeline of Next-IOᵀᴹ programs.
Fig.2 The timeline of Next-IOᵀᴹ programs.
 Download our brochure
Download our brochure