Next-IO™ Anti-CD160 Monoclonal Antibody Program
Creative Biolabs is a CRO-based pharmaceutical company that dedicates to developing novel generation antibodies to treat cancers or other diseases. Our mission is to help self and our partners develop potential novel therapeutics to help all patients in need. Now we initiate various Next-IO™ programs targeting a wide range of targets. All available for your flexible choice. Our anti-CD160 monoclonal antibody program is one of them with an aim to develop therapeutic monoclonal antibodies that could recognize and potentially, against CD160.
CD160 in T Cell
As one of the checkpoint inhibitors, CD160 is a ligand for Herpes Virus Entry Mediator (HVEM). Engagement of CD160 and HVEM (TNFRSF14) was demonstrated to be the mediator to inhibit CD4 T cell proliferation and TCR mediated signaling. It is believed targeting HVEM/LIGHT/BTLA/CD160 pathway can reverse the T cell immune function.
CD160 in NK Cell
CD160 is expressed on activated NK cells and plays an essential role in NK cell-mediated IFN-γ production. IFN-γ secretion by NK cells is important for NK-dependent tumor killing, and by reverse thinking, we believe targeting CD160 will be potential therapeutics in cancer management.
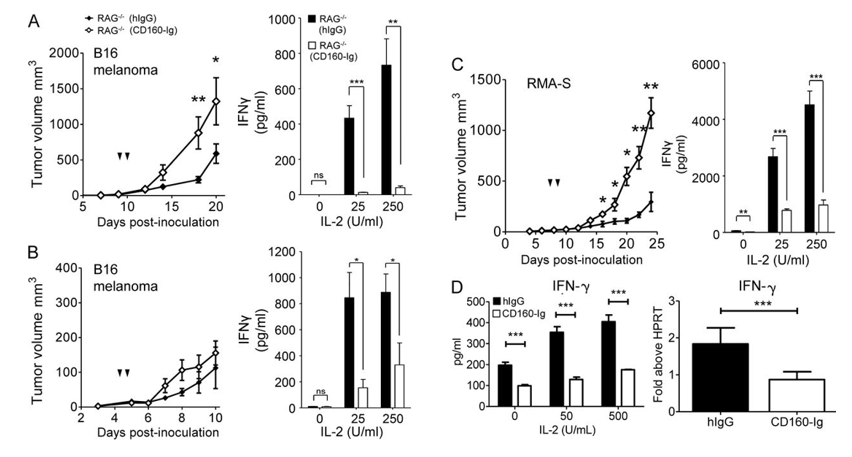 Fig.1 CD160 functionally regulates the NK response to tumor.1
Fig.1 CD160 functionally regulates the NK response to tumor.1
CD160 in B-cell Malignancies
CD160 is a tumor-specific antigen identified in different types of B cell malignancies. In one published study, CD160 expression is found at 98% in chronic lymphocytic leukemia (CLL) patients; 100% in hairy cell leukemia (HCL) patients; 15% in mantle cell lymphoma (MCL) patients; 16% in other B-LPD patients. For the reason that CD160 does not exist in normal B cells, we believe CD160 will be a novel target for the treatment of B cell malignancies.
CD160 in Blood Vessels
CD160 does not form in blood vessels of normal tissues. However, while in solid tumors, the expression of CD160 is detected at the surface of endothelial cells in newly formed blood vessels. CD160 is believed to be able to block tumor vascularisation, which inhibits tumor growth and metastasis.
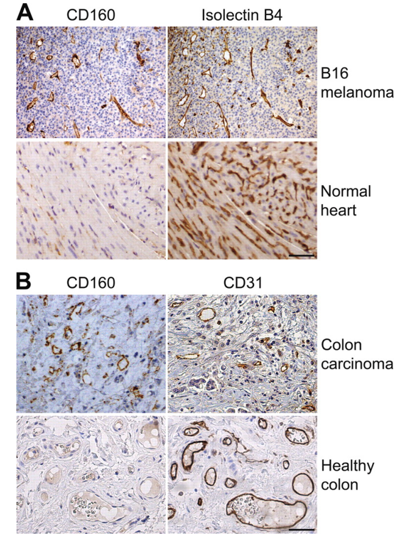 Fig.2 Expression of CD160 in tumor blood vessels but not in the blood vessels of healthy tissues.2
Fig.2 Expression of CD160 in tumor blood vessels but not in the blood vessels of healthy tissues.2
Our Next-IO™ anti-CD160 Program
Considering the diverse nature of CD160 in immunity and oncology, our anti-CD160 program will focus on the following 4 strategies.
Strategy 1
The anti-CD160 monoclonal antibody will be first identified and then optimized to 1) block the checkpoint inhibition and 2) reverse the T cell anti-tumor immune function.
Strategy 2
Then anti-CD160 monoclonal antibody will be first identified and then optimized to 1) stimulate IFNγ production and 2) reactivate the NK cell-mediated anti-tumor immune responses.
Strategy 3
The anti-CD160 monoclonal antibody will be first identified and then optimized to 1) recognize the CD160 expressed B cell malignancies and 2) trigger antibody-dependent cell cytotoxicity (ADCC) to attack tumor cells.
Strategy 4
The anti-CD160 monoclonal antibody will be first identified and then optimized to 1) target CD160 expressed endothelial cells of blood vessels in and around solid tumors and 2) block tumor vascularisation.
For combination therapies, please feel free to reach out to our scientists for additional assistance.
Clinical Trials
So far, not drug anti-CD160 drug is approved, and only two known studies are ongoing in clinical trials. Studies on anti-CD160 are still in an early stage. We believe our program of discovering and researching anti- CD160 is at the cutting edge of the field and have certain marking and sale potential.
Program Planning and Management
With extensive experience in the discovery and development of therapeutic antibodies, Creative Biolabs is dedicated to providing Next-IO™ programs more efficiently and committed to delivering the finalized program to our partners within about 1.5 years. The accurate timeline will be determined on a case-by-case basis. Here is a draft timeline for your glance.
 Fig.3 The timeline of Next-IOᵀᴹ programs.
Fig.3 The timeline of Next-IOᵀᴹ programs.
Collaboration
Creative Biolabs is excited to offer years of valuable CRO experience to our partners and achieve more strategic. We are committed to maximizing the result of our programs in a timely manager. No matter you have an idea or not at this moment, our scientists will tailor the program per the needs of you and your team. As a leading CRO company, we believe our collaboration will unleash the creative spirit and help you achieve greater success in immuno-oncology.
If you are interested in our programs, please feel free to contact us for more details.
References
-
Tu, Tony C., et al. "CD160 is essential for NK-mediated IFN-γ production." Journal of Experimental Medicine 212.3 (2015): 415-429.
-
Chabot, Sophie, et al. "A novel antiangiogenic and vascular normalization therapy targeted against human CD160 receptor." Journal of Experimental Medicine 208.5 (2011): 973-986.
For Research Use Only | Not For Clinical Use


 Fig.1 CD160 functionally regulates the NK response to tumor.1
Fig.1 CD160 functionally regulates the NK response to tumor.1
 Fig.2 Expression of CD160 in tumor blood vessels but not in the blood vessels of healthy tissues.2
Fig.2 Expression of CD160 in tumor blood vessels but not in the blood vessels of healthy tissues.2
 Fig.3 The timeline of Next-IOᵀᴹ programs.
Fig.3 The timeline of Next-IOᵀᴹ programs.
 Download our brochure
Download our brochure