This program aims to develop anti-CD19 × CD28 therapeutic Bispecific Antibody for immuno-oncology.
T cell-based cancer immunotherapies, including chimeric antigen receptor T cells, immune checkpoint inhibitors, and T cell redirecting bispecific antibodies (TRBAs), have been tested successfully on patients. Recognizing two different epitopes on T cells and cancer cells, Bispecific Antibody has attracted increasing attention as a novel cancer immunotherapy. Instead of the traditional BiTEs, which rely on CD3 signaling without providing co-stimulation, the development of our CD28 Bispecific Antibody highlights the co-stimulation pathway of T cell activation.
CD19 × CD28
CD19, a B cell-specific member of the immunoglobulin superfamily, is a key component of the BCR complex. Expressed during the most stages of B cell development until plasma cell differentiation, CD19 positively regulates the intrinsic signaling threshold and serves as a costimulatory molecule for amplifying BCR signaling and downstream B-cell proliferation.
CD28 is a differentiation antigen expressed on thymocytes and most mature T cells, including all CD4 T cells and CD8 T cells with cytolytic activity. Identified as the "T cell co-stimulation", CD28 ligation can enhance the production of various cytokines, such as IL-2, IL-4 and IL-10.
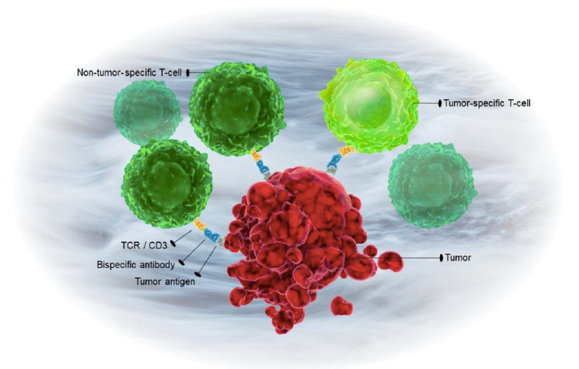 Fig.1 Bispecific Antibody for T-cell redirection.1
Fig.1 Bispecific Antibody for T-cell redirection.1
Published Data
To the best of our knowledge, anti-CD19 × CD28 therapeutic Bispecific Antibody has not been studied. Efficacy, safety, and combination strategies for anti-CD19 × CD28 therapeutic Bispecific Antibody have not been determined. Here, we show the importance of CD28 BITE, which acts as a co-receptor for maximal anti-tumor efficacy:
-
ROR1/CD28 Bispecific Antibody were ineffective alone but significantly augmented the cytotoxic effect of ROR1/CD3 BiTE.
-
ROR1/CD28 Bispecific Antibody significantly augmented the cytotoxic effect of CD19/CD3 BiTE.
-
PD-L1/CD28 Bispecific Antibody can reverse checkpoint inhibition into T-cell activation to overcome Bispecific Antibody resistance.
Indication
Based on the published data, we learn that CD19 is highly expressed in B cell malignances (including most non-Hodgkin's lymphomas (NHL), some leukemias, and myelomas), of which 85% are expressed in NHL. Therefore, our program primarily focuses on the development of therapeutic antibodies against the high-risk NHL.
Clinical Trials under Progress
Currently, there are NO clinical studies on the novel combination therapy. While our program focuses on CD28 Bispecific Antibody antibodies, future efforts will develop simultaneous multiple interaction T-cell engaging (SMITE) bispecific pairs targeting other co-receptor signaling pathways. In this case, our program will be the pioneer in the field.
With extensive experience in providing CRO services, we are confident about streamlined end-to-end program development. For each program, we are committed to developing the complete program to our partners from antibody discovery, engineering, optimization, to pre-clinical studies. Periodic progress will be delivered to our clients for timely communications.
 Fig.2 The timeline of Next-IO™ programs.
Fig.2 The timeline of Next-IO™ programs.
Creative Biolabs is looking for potential partners (including pharma or biotech firms) to co-develop anti-CD19 × CD28 Bispecific Antibody program. Our scientists are dedicated to bringing together years of valuable creation experience and research to explore strategic collaborations with our partner. This business strategy will enable both of us to proceed through IND and go beyond all stages of clinical trials.
If you are interested in our program, please feel free to contact us to learn more details about the cooperation. Looking forward to working with you in the near future.



 Fig.1 Bispecific Antibody for T-cell redirection.1
Fig.1 Bispecific Antibody for T-cell redirection.1

 Fig.2 The timeline of Next-IO™ programs.
Fig.2 The timeline of Next-IO™ programs.

 Download our brochure
Download our brochure