Next-IO™ Anti-CD94/NKG2A Monoclonal Antibody Program
For a decade, Creative Biolabs is dedicated to providing antibody engineering services to many clients in need. Empowered by our comprehensive platforms and portfolios, our scientists are at the top in the field and experience in discovering and developing novel therapeutic. Next-IO™ Anti-CD94/NKG2A Monoclonal Antibody Program aims to develop novel therapeutic antibody candidates for cancer patients in need.
Background
Natural killer (NK) cell plays a critical role in innate immune responses. The name is given because of its ability to induce rapid cytotoxicity against infected cells without prior sensitization. The cytotoxicity of NK cell is inhibited when human leucocyte antigen (HLA) is expressed on target cells, i.e self-tolerance of NK cells. This mechanism helps NK cells from attacking our own cells. In patients with cancer, HLA is highly expressed in the system and cytotoxicity from NK cells is inhibited. In addition, HLA is the ligand of checkpoints expressed on NK cells, for e.g. KIRs, CD94/NKG2A heterodimer, and etc. In recent years, cancer immunotherapy made profound progress and we believe, as a checkpoint of NK cell, CD94/NKG2A is a potentially promising therapeutic target to be studied further.
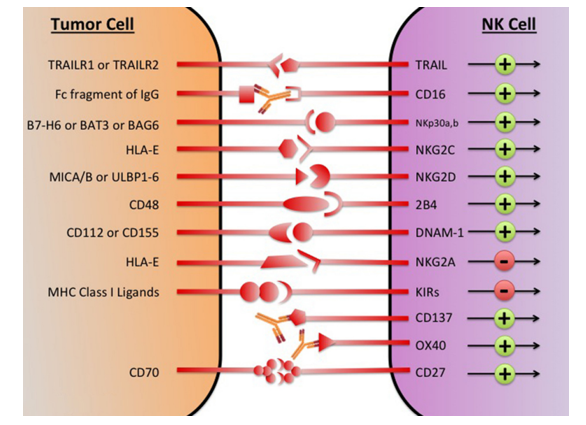 Fig.1 The major NK cell receptors that are potential immunotherapeutic targets.1
Fig.1 The major NK cell receptors that are potential immunotherapeutic targets.1
Our Anti-CD94/NKG2A Antibody Program
Our anti-NKG2A mAb program aims to develop a novel therapeutic mAb for cancer treatment. We are dedicated to promoting the program to the pre-IND stage with the help of partners. Here are features of NKG2A working as the potential therapeutic target.
-
Engagement of CD94/NKG2A by HLA-E can transduce inhibitory signals, which later can inhibit immune responses of NK cells. In other works, it can cause decreased cytotoxicity and cytokine production in NK cells;
-
CD94/NKG2A and HLA-E are correlated with poor prognosis in patients with cancer;
-
The expression level of NKG2A is higher in head and neck squamous cell cancers (HNSCC) compared with non-tumor NK cells;
-
Expression of NKG2A is correlated with poor prognosis and impaired NK cell immune functions;
-
Blocking NKG2A can partially restore the cytotoxicity of NK cells.
Other than monoclonal antibody developments, if you are interested in combination strategies, or other antibody modalities, such as bispecific antibody, etc., please feel free to reach out and we’d glad to be of assistance.
Published Data
From the data, we learn that the NKG2A was identified works as a potential therapeutic target for cancer immunotherapy. For any additional assistance, please feel free to reach out to our scientists.
Program Planning and Management
We have extensive experience in performing comprehensive program developments and effective problem-solving. For our Next-IO™ programs, we are committed to promoting the program to the pre-IND stage within about 1.5 years. The accurate timeline will be determined on a case-by-case basis. Here is a draft timeline for your glance.
 Fig.3 The timeline of Next-IOᵀᴹ programs.
Fig.3 The timeline of Next-IOᵀᴹ programs.
Collaboration
Creative Biolabs is seeking our potential partners to develop the novel therapeutic mAbs against CD94/NKG2A receptors together. With the help of our advanced platforms and senior scientists, we are committed to pushing the program forward.
If you are interested in our Next-IO™ programs, please feel free to contact us for detailed communication.
References
-
Chester, Cariad et al. "Natural killer cell immunomodulation: Targeting activating, inhibitory, and co-stimulatory receptor signaling for cancer immunotherapy." Frontiers in immunology. 6 601.
-
Gillard-Bocquet, Mélanie et al. "Lung tumor microenvironment induces specific gene expression signature in intratumoral NK cells." Frontiers in immunology. (2013) 4 19.
For Research Use Only | Not For Clinical Use


 Fig.1 The major NK cell receptors that are potential immunotherapeutic targets.1
Fig.1 The major NK cell receptors that are potential immunotherapeutic targets.1
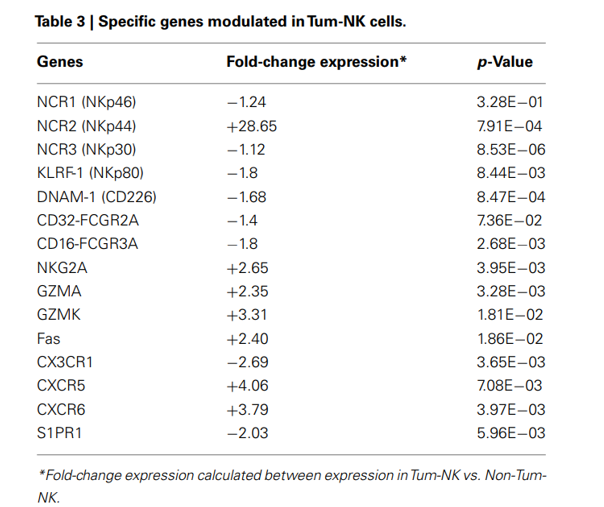 Fig.2 NKG2A is upregulated in tumor-NK cells.2
Fig.2 NKG2A is upregulated in tumor-NK cells.2
 Fig.3 The timeline of Next-IOᵀᴹ programs.
Fig.3 The timeline of Next-IOᵀᴹ programs.
 Download our brochure
Download our brochure