Next-IO™ Anti-CD96 Therapeutic Monoclonal Antibody Program
About This Program
This program aims to develop anti-CD96 therapeutic monoclonal antibody for immuno-oncology.
CD96 is a novel target for cancer immunotherapy. It regulates NK cell effector function and metastasis. Studies have shown that blocking CD96 inhibits primary tumor growth in many experimental mouse tumor models in a CD8+ T cell-dependent manner. The anti-tumor activity of anti-CD96 treatment is independent of Fc-mediated effector function and is more effective in blocking the dual combination of many immunological checkpoints, including PD-1, PD-L1, TIGIT, and CTLA-4. In addition, CD96 was found to control the cytokine and colitis-inducing potential of Th9 cells, suggesting that CD96 inhibits CD4+ T cell effector function.
Taken together, these data indicate that CD96 is an immunological checkpoint for CD8+ T cells. Blocking CD96 in combination with other immune checkpoint inhibitors is a strategy to enhance T cell activity and inhibit tumor growth.
CD96
CD96 (TACTILE) belongs to the type I transmembrane glycoprotein of the immunoglobulin superfamily. CD96 is primarily expressed by cells of hematopoietic origin, particularly on T cells and NK cells. Like TIGIT (T cell immunoglobulin and ITIM domain), CD96 belongs to a new family of cell surface receptors that bind to ligands of the nectin and nectin-like families. CD155 (necl-5; PVR) is the major ligand for binding to CD96 and TIGIT. CD155 also binds to the activating receptor DNAM-1 (CD226).
Highlights:
-
Blocking antibodies against CD96 (anti-CD96) increased NK cell effector function, resulting in suppression of metastases and this anti-tumor activity was dependent on NK cells and DNAM-1.
-
hCD96 was reported to be upregulated in subpopulations of T-acute lymphoblastic leukemia and AML and indentifed as a potential therapeutic target in AML.
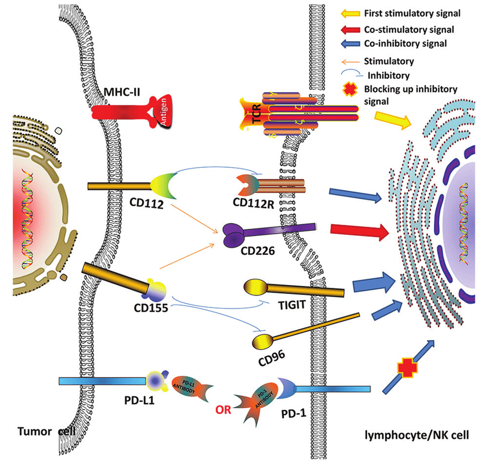 Fig.1 Association among co-stimulatory (CD226), co-inhibitory (PD-1, TIGIT, CD96, and CD112R) molecules, and their ligands (PD-L1, CD112 and CD155) in the tumor microenvironment.1
Fig.1 Association among co-stimulatory (CD226), co-inhibitory (PD-1, TIGIT, CD96, and CD112R) molecules, and their ligands (PD-L1, CD112 and CD155) in the tumor microenvironment.1
CD96 in Cancer Studies
Here are some published data about CD96 molecule work as a potential target for cancer immunotherapy.
-
Anti-CD96 therapy combines with PD-L1 or CTLA4 to enhance suppression of primary tumor growth.
-
Anti-CD96 enhances TIGIT and PD-1 blockade in a triple combination therapy
Indication
Based on the published data, CD96 is highly expressed in a variety of tumor settings, such as melanoma, ovarian cancer, and colorectal cancers. We plan to develop multiple programs (not limited to one specific type), in which CD96 is highly expressed.
Clinical Trials under Progress
To our knowledge, the anti-CD96 therapeutic monoclonal antibody has NOT been tested in any clinical trials. Our program will be a pioneer in the development of CD96 antibody.
Program Plan
We have extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years prior to entering the IND stage.
 Fig.2 The timeline of Next-IOᵀᴹ programs.
Fig.2 The timeline of Next-IOᵀᴹ programs.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop anti-CD96 therapeutic monoclonal antibody program together. Our scientists are dedicated to bringing together years of valuable experience to our partner and achieve a meaningful partnership. We hope to help both parties proceed with IND and many stages of clinical trials beyond.
If you are interested, please feel free to contact us so that we can discuss the program and other possible opportunities for collaboration. Look forward to working with you in the near future.
Reference
-
Xu Y., et al. Survival analysis with regard to PD-L1 and CD155 expression in human small cell lung cancer and a comparison with associated receptors. Oncol Lett. 2019 Mar;17(3): 2960-2968.
For Research Use Only | Not For Clinical Use


 Fig.1 Association among co-stimulatory (CD226), co-inhibitory (PD-1, TIGIT, CD96, and CD112R) molecules, and their ligands (PD-L1, CD112 and CD155) in the tumor microenvironment.1
Fig.1 Association among co-stimulatory (CD226), co-inhibitory (PD-1, TIGIT, CD96, and CD112R) molecules, and their ligands (PD-L1, CD112 and CD155) in the tumor microenvironment.1
 Fig.2 The timeline of Next-IOᵀᴹ programs.
Fig.2 The timeline of Next-IOᵀᴹ programs.
 Download our brochure
Download our brochure