About This Program
This program aims to develop anti-DLL3 therapeutic monoclonal antibody for immuno-oncology.
Rationale for the program:
-
Delta-like ligand 3 (DLL3), an inhibitory Notch pathway ligand, is highly upregulated and expressed on the cell surface of small cell lung cancer (SCLC) and other high-grade neuroendocrine tumors.
-
Notch signaling pathway is down-regulated during neuroendocrine tumor growth and DLL3 expression may contribute to its inhibition.
-
DLL3 expression is regulated by achaete-scute homolog-1 (ASCL1), which is a protein required by the normal development of pulmonary neuroendocrine cells and a carcinogenic driver in SCLC.
-
In preclinical models, DLL3 expression promotes SCLC migration and invasion through a mechanism involved in the control of the epithelial-mesenchymal transition Snail protein.
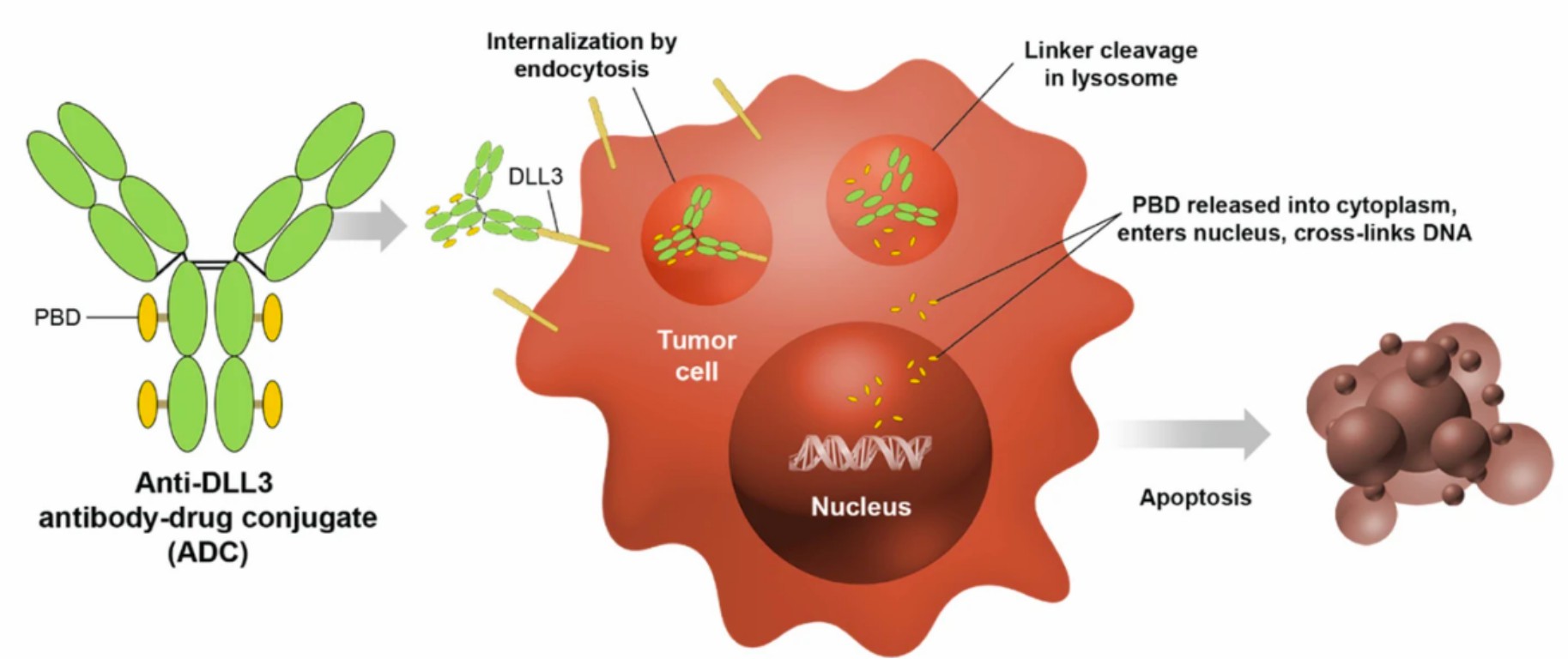 Fig.1 Internalization of the ADC
Fig.1 Internalization of the ADC
(DLL3-targeted antibody-drug conjugate) to lysosomes leads to
the cleavage of the linker, release of the toxin, and apoptosis.1
DLL3
Delta-like protein 3 (DLL3) is a key mediator in cell development that inhibits the activation of the Notch pathway in cis and trans by redirecting or retaining Notch or Notch-activation-ligand DLL1 in late endosomal/lysosomal compartments or Golgi, respectively. In addition to the expression on the surface of SCLC tumor cells, DLL3 is also expressed in the neuroendocrine origin of other tumor types, including melanoma, glioblastoma multiforme, small cell bladder cancer, prostate cancer, and neuroendocrine lung tumors.
DLL3 in Cancer Studies
Here are some published data about DLL3 working as a potential target for cancer immunotherapy.
-
DLL3 expression is associated with worse overall survival (OS) compared to DLL3-negative cases in the prostate cancer model.
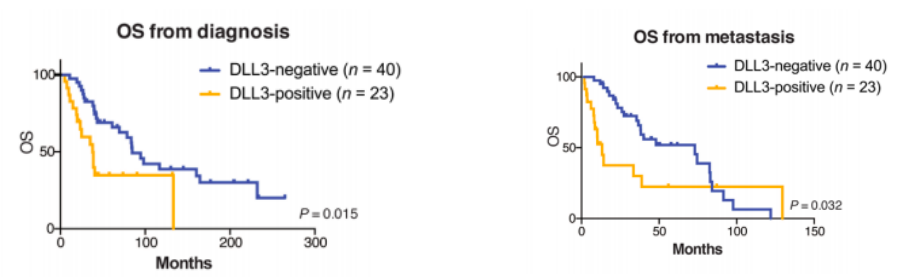 Fig. 2 OS of patients with prostate cancer evaluated from the time of prostate cancer diagnosis and the time of metastasis.2
Fig. 2 OS of patients with prostate cancer evaluated from the time of prostate cancer diagnosis and the time of metastasis.2
-
DLL3 drug-conjugates inhibits tumor growth in DLL3-expressing prostate tumors.
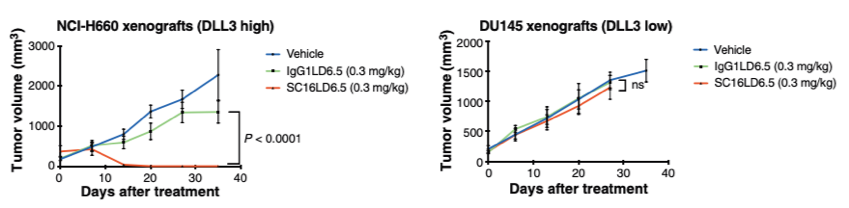 Fig. 3 DU145 and NCI-H660 tumor volume measurements after dose treatments.2
Fig. 3 DU145 and NCI-H660 tumor volume measurements after dose treatments.2
Ongoing Clinical Trials
-
Currently, several anti-DLL3 therapeutic monoclonal antibodies are being evaluated in clinical trials. Cumulative clinical data demonstrate its important role in cancer progression; however, safety, efficacy, and combination strategies require further confirmation.
-
Sufficient rationality for us to believe that DLL3 is still a compelling target for cancer immunotherapy. In an effort to optimally leverage DLL3-mediated immune response, our next generation of DLL3 targeting treatment attempts to explore combination therapy trials by involving other immunomodulatory agents.
Program Planning and Management
Creative Biolabs has extensive knowledge in end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.
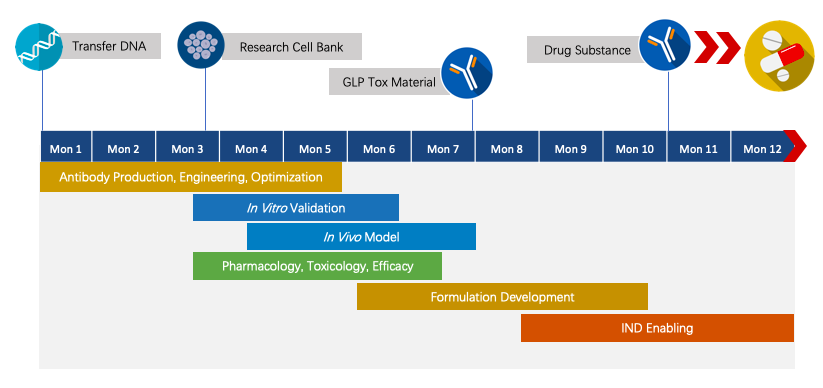 Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop anti-DLL3 therapeutic monoclonal antibody program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership. For any partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners advance their programs with more chance to succeed. Look forward to cooperating with you in the near future.


 Fig.1 Internalization of the ADC
Fig.1 Internalization of the ADC  Fig. 2 OS of patients with prostate cancer evaluated from the time of prostate cancer diagnosis and the time of metastasis.2
Fig. 2 OS of patients with prostate cancer evaluated from the time of prostate cancer diagnosis and the time of metastasis.2
 Fig. 3 DU145 and NCI-H660 tumor volume measurements after dose treatments.2
Fig. 3 DU145 and NCI-H660 tumor volume measurements after dose treatments.2
 Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Fig.4 Project pipeline management of therapeutic monoclonal antibody.
 Download our brochure
Download our brochure