This program aims at developing therapeutic monoclonal antibodies against the target – Glucocorticoid-induced TNFR-related protein (GITR).
GITR is a promising target for immunotherapy due to its ability to promote T-cell effector (Teff) functions and hamper the regulation of T-cell (Treg) suppression. Multiple studies have shown that agonist anti-GITR antibodies are very potent in their anti-tumor activity. For this reason, the highlight of this program is to use GITR as a potential novel therapeutic target in the field of immune-oncology.
GITR
GITR, also referred to as TNFRSF18, is a type I transmembrane protein from the tumor necrosis factor receptor superfamily. GITR is preferably expressed in natural killer (NK) and T cells, especially in Treg cells. Based on data from mouse tumor studies, the therapeutic activity of GITR agonist is associated with the reduction and functional modulation of intra-tumor Treg cells, and enhanced anti-tumor CD8+ T cell functions. Multiple studies have shown that:
-
Expression of GITR is significantly increased upon T-cell activation and lead to enhanced cellular and humoral immunity.
-
GITR-GITRL interaction provides a co-stimulatory signal to both CD4+ and CD8+ naïve T cells, enhancing their proliferation and effector function, particularly in the case of suboptimal T cell receptor (TCR) stimulation.
-
Tregs cells constitutively express GITR, while GITR ligand or agonistic antibody can activate GITR
signaling and inhibit the suppressive activity of Tregs.
Anti-GITR Antibody Research in Cancer Studies
Anti-GITR antibody is often used in combination with other immunology agents in the clinic settings to
improve antitumor effects. Here are the published data about GITR as a potential target for cancer
immunotherapy.
• The anti-GITR antibody DTA-1 was used for melanoma.
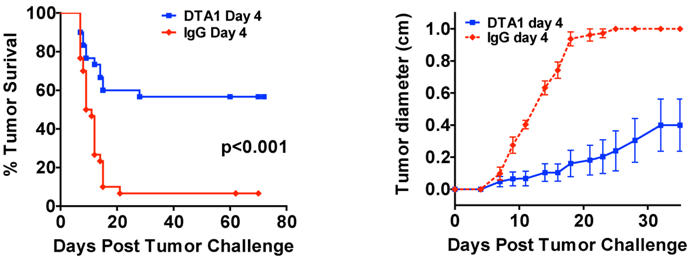 Fig.1 The anti-GITR antibody DTA-1 was used for melanoma.4
Fig.1 The anti-GITR antibody DTA-1 was used for melanoma.4
• Anti-GITR mAb plus SRS combination therapy for Glioblastoma (GBM).
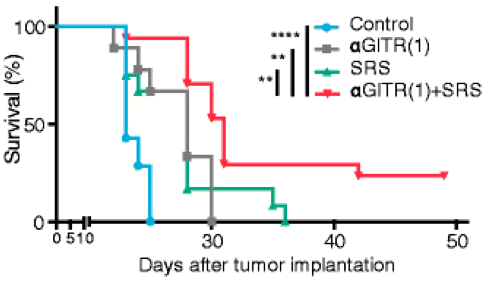 Fig.2 Anti-GITR mAb plus SRS combination therapy for Glioblastoma (GBM).3
Fig.2 Anti-GITR mAb plus SRS combination therapy for Glioblastoma (GBM).3
• Combined anti-PD-1/GITR mAb induces tumor-specific long-lasting immunity against ovarian cancer.
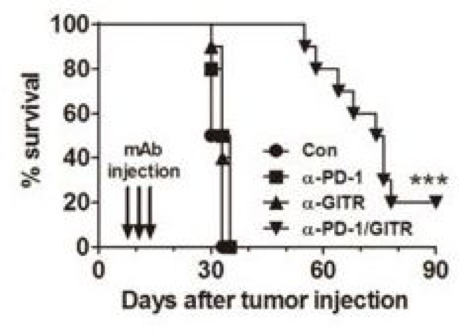
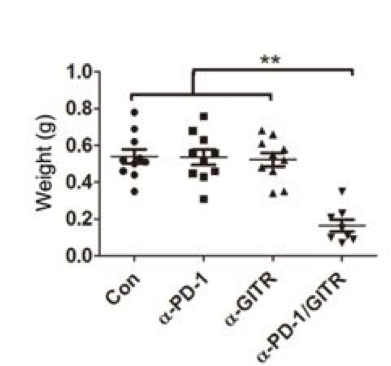
Fig.3 Combined anti-PD-1/GITR mAb induces tumor-specific long-lasting immunity against ovarian cancer.2
Indication
Based on the published data, we learn that GITR is highly expressed in different tumors, especially in solid malignancies, such as hepatocellular carcinoma, melanoma, colon carcinoma, and sarcomas. Therefore, we hope to develop multiple mAb programs that are applicable to different indications (not limited to one specific type of tumor), of which GITR is highly expressed..
Clinical Trials under Progress
Currently, several anti-GITR therapeutic monoclonal antibodies have been tested in clinical trials for several types of solid tumors. An increasing number of agonistic mAbs are getting confirmed on its role to stimulate T cells, and early clinical trial data have also shown their great promise. However, so far, there are no significant efficacy data from phase II or phase III clinical trials.
In this case, GITR still owns a promising market prospect in the future. In particular, there is increasing interest in combining anti-GITR with other agents, and testing their synergistic ability in various cancers.
With our extensive experience in providing CRO services, we are confident in providing streamlined end-to-end program development. We are committed to developing a complete program, which tailors to the needs of our partners, from antibody discovery, engineering, optimization, to pre-clinical studies. Periodic progress reports will be delivered to our clients for effective, smooth and timely communication.
 Fig.4 The timeline of Next-IO™ programs.
Fig.4 The timeline of Next-IO™ programs.
Creative Biolabs is looking for potential partners (including but not limited to major pharma or biotech firms) to develop anti-GITR monoclonal antibody program together. Our scientists are dedicated to bringing years of valuable experience to our partner and enabling a meaningful partnership. By using this strategic collaborations, we wish to help both sides to proceed with IND and all stages of clinical trials.
If you are interested in our program, please feel free to contact us to learn more
details about the
cooperation. Looking forward to working with you in the near future.



 Fig.1 The anti-GITR antibody DTA-1 was used for melanoma.4
Fig.1 The anti-GITR antibody DTA-1 was used for melanoma.4
 Fig.2 Anti-GITR mAb plus SRS combination therapy for Glioblastoma (GBM).3
Fig.2 Anti-GITR mAb plus SRS combination therapy for Glioblastoma (GBM).3



 Fig.4 The timeline of Next-IO™ programs.
Fig.4 The timeline of Next-IO™ programs.

 Download our brochure
Download our brochure