Next-IO™ Anti-GPVI Therapeutic Monoclonal Antibody Program
About This Program
This program aims to develop anti-GPVI therapeutic monoclonal antibody for immuno-oncology.
Tumor vascular integrity is essential for tumor growth, thus affecting tumor progression. Previous studies have determined that platelets are targets of potential anti-tumor therapy and major regulators of tumors. Studies have also shown that platelet glycoprotein (GP) VI (GPVI) is a key regulator of vascular integrity in tumor growth, which can induce tumor hemorrhage and reduce tumor growth without causing systemic bleeding complications. These data fully indicate that GPVI could be a promising target for next generation cancer immunotherapy.
GPVI
Glycoprotein (GP) VI, a platelet receptor for collagen, laminin and fibrin, is a type 58 transmembrane protein with 58-60 kDa molecular weight. It mainly regulates a variety of platelet functions, including adhesions, activation, aggregation, and procoagulant activity.
Highlight functions of GPVI:
-
For many years, GPVI has been considered a potentially safe antithrombotic target
-
Important in maintaining vascular integrity
-
Inhibition of GPVI rapidly induces severe tumor hemorrhage, reduces tumor growth, and increases intratumoral accumulation of co-administered chemotherapeutic drugs, resulting in a significant increase in anti-tumor effects.
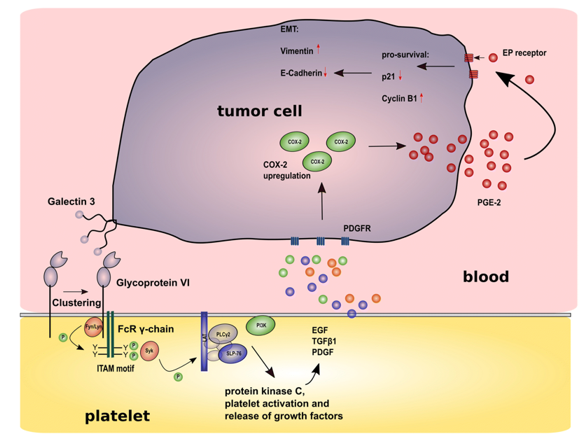 Fig.1 Impact of the interaction between GPVI and galectin 3 on cancer metastasis. (Schlesinger, 2018)
Fig.1 Impact of the interaction between GPVI and galectin 3 on cancer metastasis. (Schlesinger, 2018)
GPVI in Cancer Studies
Here are some published data about GPVI molecule work as a potential target for cancer immunotherapy.
-
Increased bleeding in TrampC1 (prostate cell ) and AT-3 (breast cancer cell) primary tumor in GPVI deficient animals and upon GPVI-blockade.
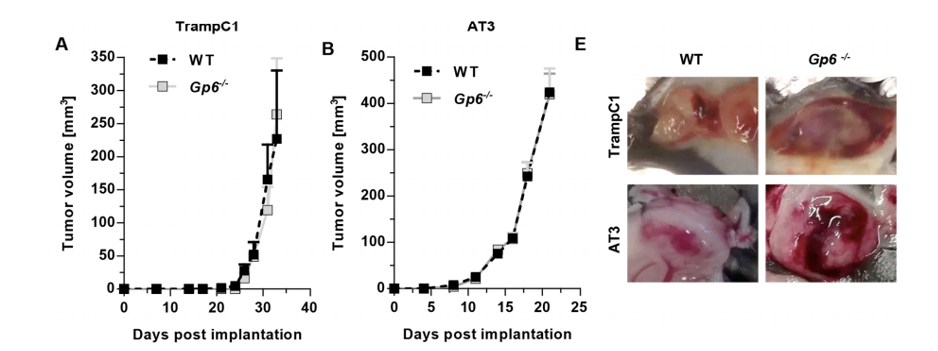 (Volz, 2019)
(Volz, 2019)
-
Anti-GPVI antibody treatment together with rt-PA administration improves stroke outcome in a transient middle cerebral artery occlusion (tMCAO) model.
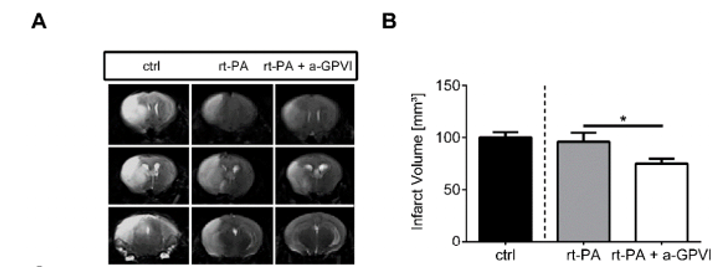 (Schuhmann, 2019)
(Schuhmann, 2019)
Clinical Trials under Progress
Currently, several studies of GPVI therapeutic mAbs are undergoing early clinical trials for the treatment of thromboembolic disorders. Accumulating preclinical evidence supports their clinical development. However, further studies are needed to identify the efficacy, safety, and combination strategies of GPVI therapeutic mAbs in cancer.
Looking ahead, GPVI owns a promising market prospect in the future. In particular, there are increasing interests in combining anti-GPVI with other agents, thus representing a particularly promising approach to achieving greater therapeutic success in treating various cancers.
Program Plan
We have extensive experience in end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years prior to entering the IND stage.

Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop anti-GPVI therapeutic monoclonal antibody program together. Our scientists are dedicated to bringing together years of valuable experience to our partner and achieve a meaningful partnership. By doing so, we wish to help both parties to proceed with IND and many stages of clinical trials beyond.
If you are interested, please feel free to contact us so that we can discuss the program and other possible opportunities for cooperation. Look forward to working with you in the near future.
References
-
Schlesinger, M.; et al. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018, 11(1): 125.
-
Volz, J.; et al. Inhibition of platelet GPVI induces intratumor hemorrhage and increases efficacy of chemotherapy in mice. Blood, The Journal of the American Society of Hematology. 2019, 133(25): 2696-2706.
-
Schuhmann, M.K.; et al. Targeting platelet GPVI plus rt-PA administration but not α2β1-mediated collagen-binding protects against ischemic brain damage in mice. International Journal of Molecular Sciences. 2019, 20(8).
For Research Use Only | Not For Clinical Use


 Fig.1 Impact of the interaction between GPVI and galectin 3 on cancer metastasis. (Schlesinger, 2018)
Fig.1 Impact of the interaction between GPVI and galectin 3 on cancer metastasis. (Schlesinger, 2018)
 (Volz, 2019)
(Volz, 2019)
 (Schuhmann, 2019)
(Schuhmann, 2019)

 Download our brochure
Download our brochure