Next-IO™ Anti-HCK Therapeutic Monoclonal Antibody Program
About This Program
This program aims to develop anti-HCK therapeutic monoclonal antibody in the immuno-oncology.
Rationale of the program:
-
Studies have shown that excessive HCK activation is associated with several types of leukemia.
-
Increased HCK activity is observed in many solid malignancies, including breast and colon cancer, causing decreased survival time in patients.
-
HCK contributes to the secretion of growth factors and pro-inflammatory cytokines derived from bone marrow cells and can promote macrophage polarization toward wound healing and tumor promotion.
-
In tumor-associated macrophages (TAMs), HCK stimulation promotes the formation of extracellular matrix-degrading podosomes, which enhances immunity and epithelial cell invasion.
In summary, HCK directly inhibits cancer cells growth, or indirectly inhibits the tumor transformation in the tumor microenvironment, highlighting a rationale for HCK-based cancer immunotherapy.
HCK
Hematopoietic cell kinase (HCK) is a cytoplasmic non-receptor tyrosine kinases (SFK) belongs to the SRC cytoplasmic family. HCK is mainly expressed in the bone marrow and B lymphocyte cell lineages and has nine distinct subsets: Blk, c-Src, Fyn, Fgr, Hck, Lck, Lyn, Yes and Yrk. The SFK family has proven to be a potential therapeutic target, as reflected in their roles:
-
The SFK family integrates signals from a variety of cell surface receptors, such as receptor tyrosine kinase (RTK), erythropoietin (Epo) receptor, chemokine receptor, cMET, and CXCR4, to regulate cell differentiation, growth, survival, migration, transmission, and resistance.
-
SFK is overexpressed in solid tumors and associated with poor prognosis.
-
Activation of the Src kinase family alters integrin activity and may contribute to tumor phenotype by adjusting cancer cells ability to penetrate the surrounding extracellular matrix and, thereby, metastasize.
-
Some members of SFK, LYN, FGR and HCK, are overexpressed in acute myeloid leukemia.
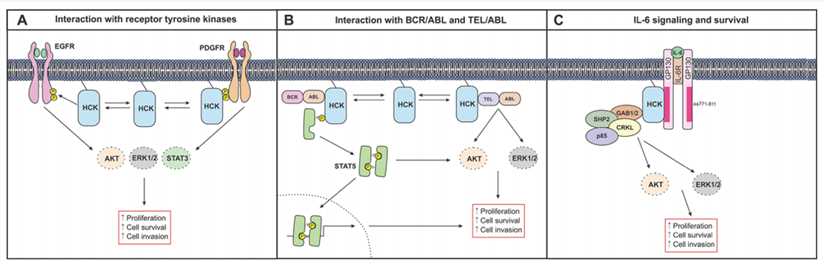 Fig.1 Simplified schematic depiction of HCK signaling in cancer cells.1
Fig.1 Simplified schematic depiction of HCK signaling in cancer cells.1
HCK in Cancer Studies
Here are some published data about HCK working as a potential target for cancer immunotherapy.
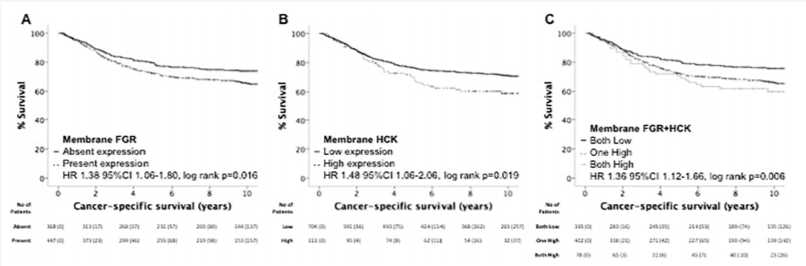 Fig.2 Activated FGR and HCK associate with poor prognosis in patients undergoing potentially curative resection of colorectal cancer.2
Fig.2 Activated FGR and HCK associate with poor prognosis in patients undergoing potentially curative resection of colorectal cancer.2
-
Src family kinases, HCK, and FGR are associated with local inflammation and tumor progression in colorectal cancer.
-
HCK inhibitor (SU) can enhance the anti-tumor effects of Immunotoxins.
Ongoing Clinical Trials
Program Planning and Management
We have extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.
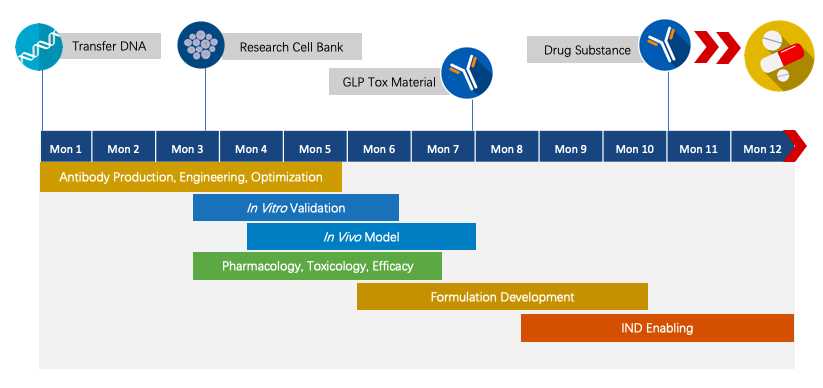 Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop anti-HCK therapeutic monoclonal antibody program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership. For any partners interest in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners advance their programs with more chance to succeed. Look forward to cooperating with you in the near future.
References
-
Poh, AR.; et al. Hematopoietic cell kinase (HCK) as a therapeutic target in immune and cancer cells. Oncotarget. 2015,6(18):15752.
-
Roseweir, AK.; et al. Src family kinases, HCK and FGR, associate with local inflammation and tumor progression in colorectal cancer. Cellular Signalling. 2019,56:15-22.
-
Liu, X F.; et al. Antitumor effects of immunotoxins are enhanced by lowering HCK or treatment with SRC kinase inhibitors. Molecular cancer therapeutics. 2014,13(1):82-89.
For Research Use Only | Not For Clinical Use


 Fig.1 Simplified schematic depiction of HCK signaling in cancer cells.1
Fig.1 Simplified schematic depiction of HCK signaling in cancer cells.1
 Fig.2 Activated FGR and HCK associate with poor prognosis in patients undergoing potentially curative resection of colorectal cancer.2
Fig.2 Activated FGR and HCK associate with poor prognosis in patients undergoing potentially curative resection of colorectal cancer.2
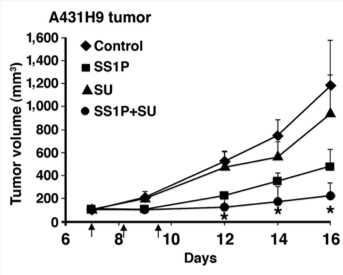 Fig.3 Src family inhibitor enhanced SS1P toxicity in mouse xenograft model.3
Fig.3 Src family inhibitor enhanced SS1P toxicity in mouse xenograft model.3
 Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Fig.4 Project pipeline management of therapeutic monoclonal antibody.
 Download our brochure
Download our brochure