About This Program
This program aims to develop anti-folate receptor alpha (SIRPα) therapeutic monoclonal antibody for immuno-oncology.
Signaling protein alpha (SIRPα), also known as differentiation cluster (CD) 172a or Src homology 2, is a cell surface receptor expressed on bone marrow and hematopoietic stem cells and neurons. Accumulated data indicates that SIRPα plays an important role in cell signaling as the negative regulator for phosphatidylinositol 3-kinase signaling and mitogen-activated protein kinase pathways. SIRPα is involved in various cancers including prostate cancer, breast cancer, and liver cancer, astrocytoma, and myeloid malignancies. Recently, blocking CD47, an anti-phagocytic signal as the SIRPα ligand, has been shown to have promising therapeutic effects, highlighting the rationale to advance SIRPα/CD47 axis-based cancer immunotherapy.
SIRPα
SIRPα is a cell surface receptor mainly expressed in monocytes, granulocytes, dendritic cells, and hematopoietic stem cells. But the expression in bone marrow cells and neurons are also permitted. Highlighting features as follows:
-
SIRPα significantly inhibits the growth of anchorage-independent cell.
-
Binding of SIRPα to its ligand CD47 can transduce down-regulated signal that inhibits phagocytosis.
-
SIRPα overexpression may result in reduced reactivity to tyrosine kinase ligands, such as epidermal growth factor (EGF), platelet-derived growth factor and insulin.
-
SIRPα downregulation activates downstream pathways that can promote cell proliferation, transformation, migration and invasion.
-
Cell type and its expression levels may determine the role of SIRPα.
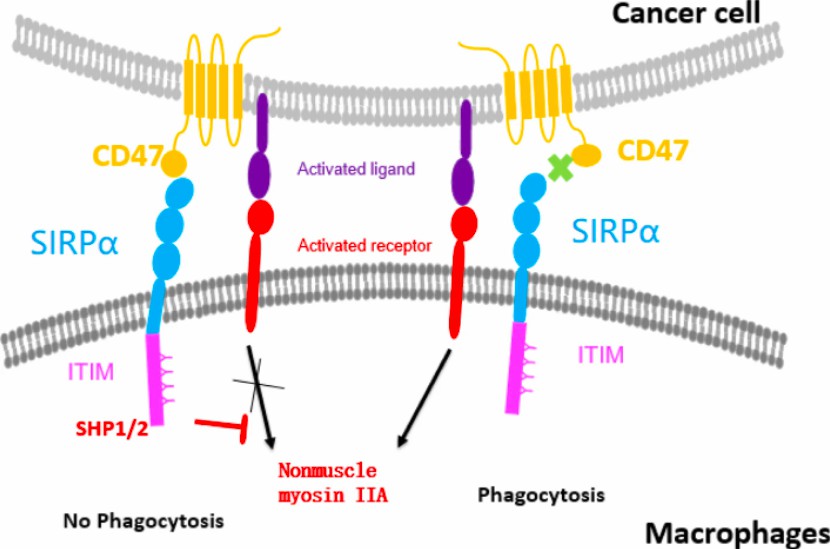 Fig.1 Biology of CD47/SIRPα interaction.4
Fig.1 Biology of CD47/SIRPα interaction.4
SIRPα in Cancer Studies
Here are some published data about SIRPΑ working as a potential target for cancer immunotherapy.
-
Anti- SIRPα antibody (KWAR23) / anti-CD20 antbody (Rituximab) combination treatment shows robust anti-tumor efficacy.
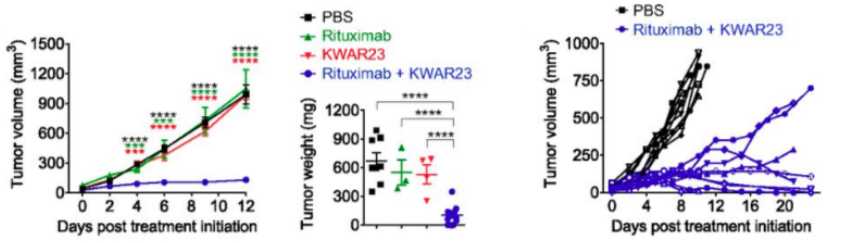 Fig.2 Tumor volume, weight and individual tumor growth curves in SRG mice treated with indicated treatments.3
Fig.2 Tumor volume, weight and individual tumor growth curves in SRG mice treated with indicated treatments.3
-
Combination therapy with anti- SIRPα antibody (MY-1) and rituximab, or a mAb against PD-1, shows greater efficacy on down-regulation on tumor growth in vivo.
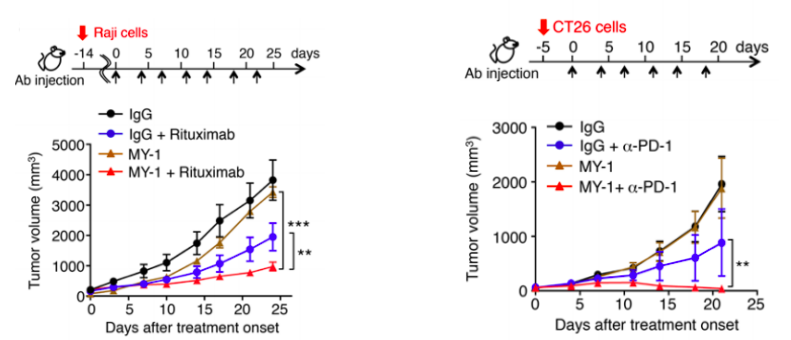 Fig.3 Mice were injected s.c. with Raji/CT26 cells and then treated with the indicated combinations of Abs according to the indicated schedule.5
Fig.3 Mice were injected s.c. with Raji/CT26 cells and then treated with the indicated combinations of Abs according to the indicated schedule.5
Indication
SIRPα is widely expressed in many tissues and normally, down-regulated in various cancers. Down-regulation activates downstream signaling pathways, ultimately leading to an increase in cancer cell growth. Cancers with low SIRPα expression include prostate cancer, breast cancer, liver cancer, and myeloid malignancies.
|
Tissue
|
Expression Level
|
|
Prostate cancer
|
Downregulated in prostate cancer tissues and cell lines
|
|
Brain cancer
|
Expressed in 8 of 9 astrocytoma cell lines and 7 of 10 primary brain tumor biopsies
|
|
Breast cancer
|
Downregulated in breast cancer tissues
|
|
Liver cancer
|
Lower expression in hepatocellular carcinoma tissues than in matched normal tissues
|
|
Myeloid malignancies
|
Significantly suppressed in the majority of myeloid malignancies
|
Clinical Trials under Progress
-
At present, several anti- SIRPα therapeutic monoclonal antibodies are being evaluated in clinical trials. The cumulative data further confirm its role. However, efficacy, safety, and combination strategies have not yet been determined.
-
In this case, SIRPα is still a compelling target for cancer immunotherapy. In an effort to optimally leverage SIRPα/CD47 axis-based immune response, our next generation of SIRPα targeting treatment attempts to explore combination therapy trials with other immunomodulatory agents.
Program Planning and Management
We have extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before. entering the IND stage.
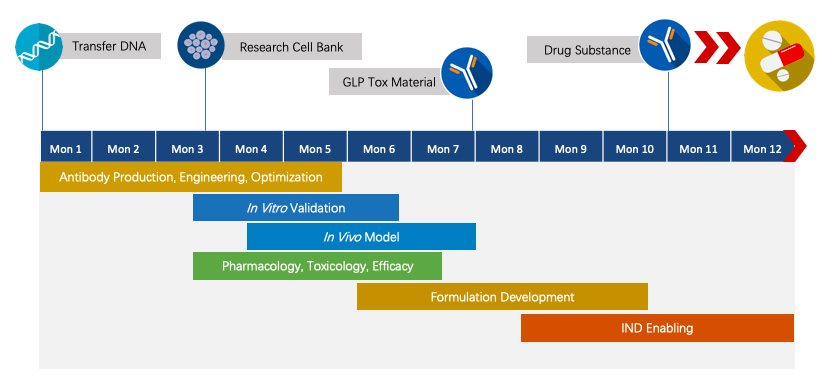 Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop anti-SIRPΑ therapeutic monoclonal antibody program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership. For any partners interest in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners advance their programs with more chance to succeed. Look forward to cooperating with you in the near future.


 Fig.1 Biology of CD47/SIRPα interaction.4
Fig.1 Biology of CD47/SIRPα interaction.4
 Fig.2 Tumor volume, weight and individual tumor growth curves in SRG mice treated with indicated treatments.3
Fig.2 Tumor volume, weight and individual tumor growth curves in SRG mice treated with indicated treatments.3
 Fig.3 Mice were injected s.c. with Raji/CT26 cells and then treated with the indicated combinations of Abs according to the indicated schedule.5
Fig.3 Mice were injected s.c. with Raji/CT26 cells and then treated with the indicated combinations of Abs according to the indicated schedule.5
 Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Fig.4 Project pipeline management of therapeutic monoclonal antibody.
 Download our brochure
Download our brochure