Next-IO™ Anti-STAT3 Therapeutic Monoclonal Antibody Program
About This Program
This program aims to develop anti-STAT3 therapeutic monoclonal antibody in the immuno-oncology. A variety of research data supports this project:
-
Controlling transcriptional 3 (STAT3) signaling pathway in epithelial cells, immune cells, and stromal cells is critical for wound healing and tissue repair.
-
Excessive STAT3 activations in cancer cells and tumor cells are considered as a neoplastic mimic of the inflammation-driven repair response, which contributes to tumor progression.
-
STAT3 promotes stem cell-like features, i.e survival, proliferation, metastatic potential, and immune escape, through classical transcriptional mechanisms. Cytoplasmic STAT3 also contributes to tumor growth through metabolism and other non-transcriptional mechanisms.
-
Many cytokines are involved in pro-inflammatory reaction in most solid malignancies to aggregate dysregulated STAT3 activity.
In summary, interference on STAT3 activity may inhibit cancer cell growth while simultaneously, enhance anti-tumor immunity. Targeting these cytokines, their cognate receptors, and associated signaling cascades shows promising therapeutic effects.
STAT3
The STAT3 signaling pathway has been extensively reviewed in the past. In normal physiological conditions, STAT3 activation is tightly controlled by endogenous inhibitors. Once upstream cytokines (e. g, IL-6) or growth factors (e. g, EGF, FGF, and VEGF) bind to cell surface receptors, STAT3 is phosphorylated and activated by JAK or Src. Activated STAT3 can further induce transcription of downstream target genes to modulate gene transcription of various cancer phenotypes.
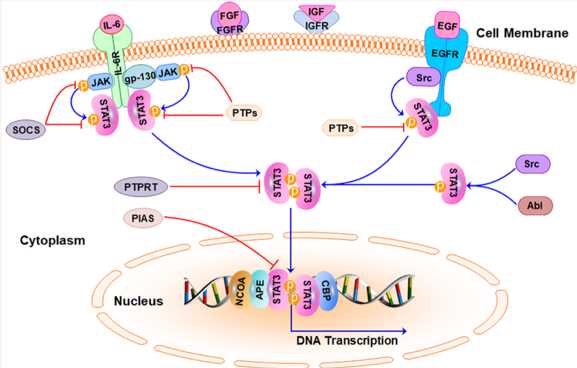 Fig.1 The STAT3 signaling pathway in cancer cells.3
Fig.1 The STAT3 signaling pathway in cancer cells.3
STAT3 in Cancer Studies
Here are some published data about STAT3 working as a potential target for cancer immunotherapy.
-
ACEE (STAT3 inhibitor) inhibits tumor growth and lung metastasis in vivo.
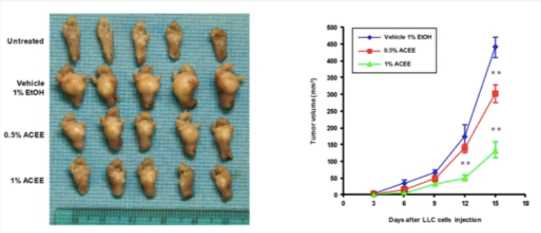 Fig.2 Primary tumors and volume of developing Lewis lung carcinoma paw tumors in vehicle and ACEE-treated mice.1
Fig.2 Primary tumors and volume of developing Lewis lung carcinoma paw tumors in vehicle and ACEE-treated mice.1
-
STAT3 inhibitor (SG-1721) exhibits therapeutic efficacy on triple-negative breast cancer (TNBC).
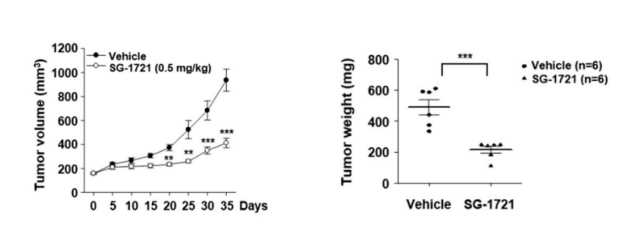 Fig.3 SG-1721 inhibits tumor growth in vivo. The tumor volumes and tumor weight was measured during the experiment.2
Fig.3 SG-1721 inhibits tumor growth in vivo. The tumor volumes and tumor weight was measured during the experiment.2
Ongoing Clinical Trials
-
Currently, several anti- STAT3 antibodies are being evaluated in clinical trials after successful testing in animal models. However, only one subset of STAR-related diseases are researched, other newly identified tumors like kidney cancer and multiple myeloma still call for further research.
-
In this case, STAT3 is still a compelling target for cancer immunotherapy. In an effort to optimally leverage STAT3-mediated immune response, our next generation of STAT3 targeting treatment attempts to explore combination therapy trials by involving other immunomodulatory agents.
Program Planning and Management
We have extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.
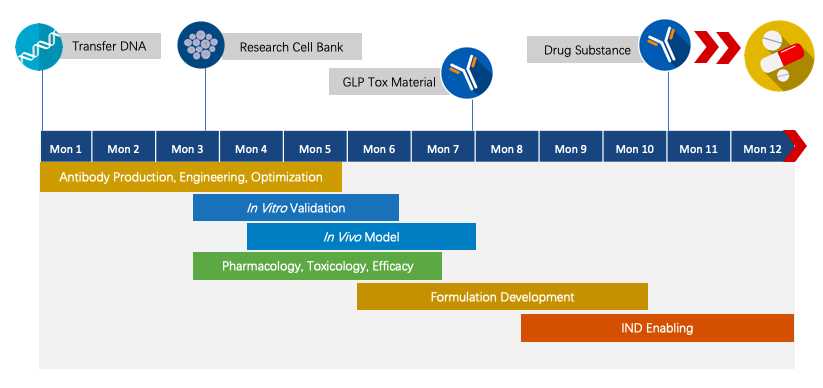 Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop anti-STAT3 therapeutic monoclonal antibody program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership. For any partners interest in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners advance their programs with more chance to succeed. Look forward to cooperating with you in the near future.
References
-
Huang, TT.; et al. Antrodia cinnamomea induces anti-tumor activity by inhibiting the STAT3 signaling pathway in lung cancer cells. Scientific Reports. 2019, 9(1):5145..
-
Hyejin, Ko.; et al. Novel Galiellalactone Analogues Can Target STAT3 Phosphorylation and Cause Apoptosis in Triple-Negative Breast Cancer. Biomolecules. 2019, 9(5):170.
-
Qin, J J.; et al. STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review. Journal of Experimental & Clinical Cancer Research. 2019, 38(1): 1-16.
For Research Use Only | Not For Clinical Use


 Fig.1 The STAT3 signaling pathway in cancer cells.3
Fig.1 The STAT3 signaling pathway in cancer cells.3
 Fig.2 Primary tumors and volume of developing Lewis lung carcinoma paw tumors in vehicle and ACEE-treated mice.1
Fig.2 Primary tumors and volume of developing Lewis lung carcinoma paw tumors in vehicle and ACEE-treated mice.1
 Fig.3 SG-1721 inhibits tumor growth in vivo. The tumor volumes and tumor weight was measured during the experiment.2
Fig.3 SG-1721 inhibits tumor growth in vivo. The tumor volumes and tumor weight was measured during the experiment.2
 Fig.4 Project pipeline management of therapeutic monoclonal antibody.
Fig.4 Project pipeline management of therapeutic monoclonal antibody.
 Download our brochure
Download our brochure