Our anti-VISTA monoclonal antibody program aims to develop monoclonal antibodies that could recognize and, potentially, against the immune checkpoint - VISTA (V-domain Ig suppressor of T cell activation). The development of such antibodies is still in the preliminary stage. We believe our program is unique and a leading program in the current biopharmaceutical market.
Background
For cancer immunotherapy, how to restore the immune response in the tumor microenvironment is the major obstacle when targeting cancer cells. Various factors may contribute to immune suppression, such as types of immune checkpoints. B7 pathway-targeting molecules are known to have played an inhibitory role in various cancers. For example, monoclonal antibodies against programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have shown to have anti-tumor efficacy, and several antibodies products are already approved by officials like FDA.
V-domain Suppressor of T Cell Activation (VISTA)
VISTA, also known as programmed death-1 homolog (PD-1H), is a member of B7-family. It is homology to PD-L1and PD-L2. VISTA has a dual activity of immune activation and T cell suppression. Scientists have already proved VISTA is expressed in tumor cells and shows immunosuppressive functions. In addition, blocking VISTA shows to improve the anti-tumor efficacy and survival rate in animal models. All these findings suggest VISTA can be the novel target for cancer immunotherapy.
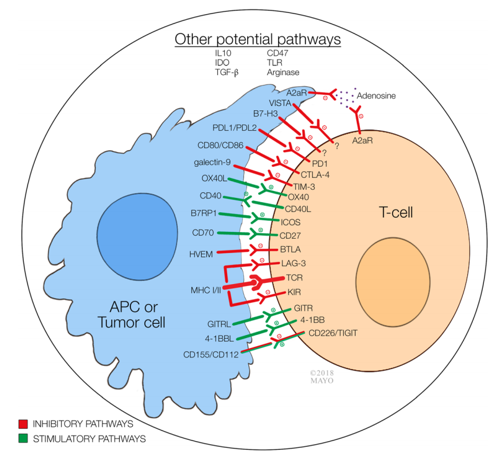 Fig.1 Immune interactions involving antigen-presenting cells or tumor cells, T cells, and tumor microenvironment.1
Fig.1 Immune interactions involving antigen-presenting cells or tumor cells, T cells, and tumor microenvironment.1
Published Data
-
High level of VISTA is expressed in patients with endometrial and ovarian cancers.
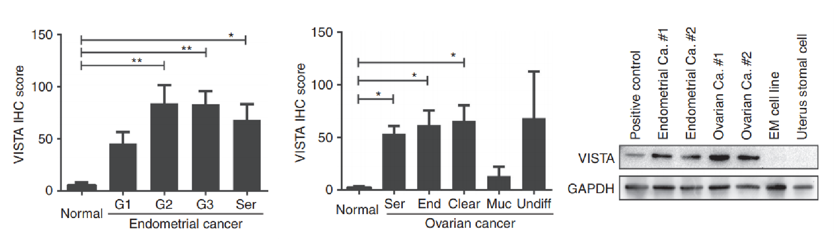 Fig.2 High level of VISTA is expressed in patients with endometrial and ovarian cancers.2
Fig.2 High level of VISTA is expressed in patients with endometrial and ovarian cancers.2
-
Silencing VISTA expression in human cancer cells restores T cell proliferation and cytokine secretion.
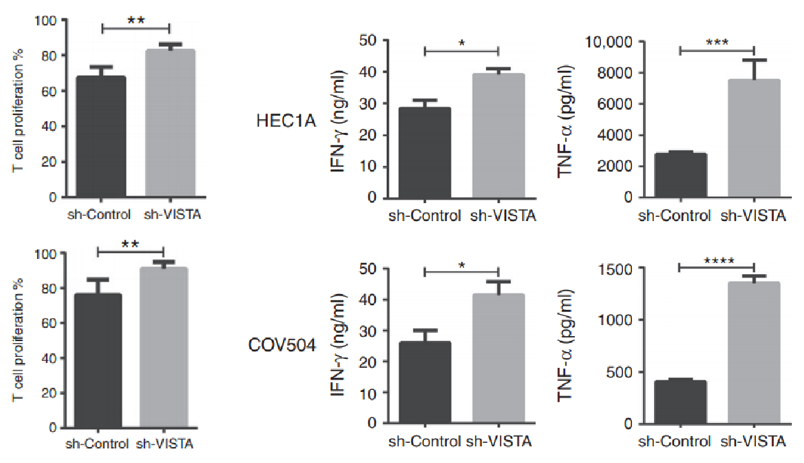 Fig.3 Silencing VISTA expression in human cancer cells restores T cell proliferation and cytokine secretion.2
Fig.3 Silencing VISTA expression in human cancer cells restores T cell proliferation and cytokine secretion.2
-
Anti-VISTA antibody treatment extends survival time in mice model.
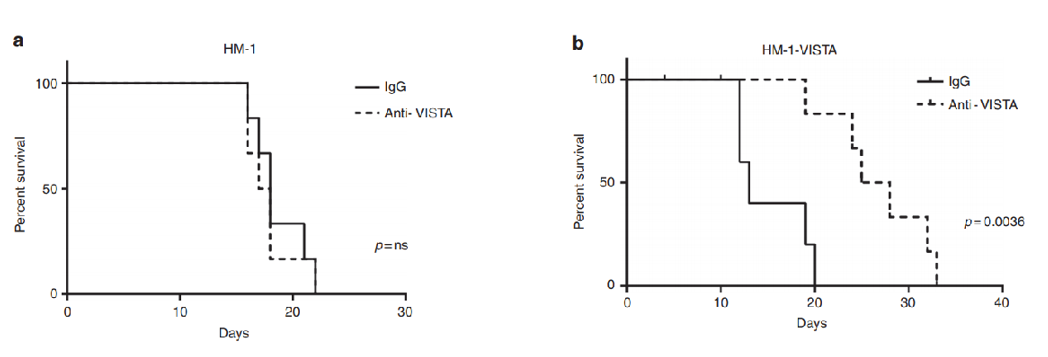 Fig.4 Anti-VISTA antibody treatment extends survival time in mice model.2
Fig.4 Anti-VISTA antibody treatment extends survival time in mice model.2
Ongoing Clinical Trials
-
Two molecules are now being tested in phase I clinical trials: JNJ-61610588, a complete human mAb against VISTA, and CA-170, an oral inhibitor of both PD-L1/PD-L2 and VISTA. Both trials are currently recruiting patients.
|
Target
|
Drug
|
Trial
|
Phase
|
Type of tumor
|
Comments
|
|
VISTA
|
JNJ-61610588
|
NCT02671955
|
I
|
Advanced tumors
|
Ongoing
|
|
CA-170
|
NCT02812875
|
I
|
Solid tumors and lymphomas
|
This molecule also inhibits PD-L1/PD-L2
|
(Marin-Acevedo, 2018)
-
VISTA is a novel checkpoint, and clinical trials to target it are still very preliminary. We believe this project will be the shining star and pose certain marketing prospects in the field.
We have extensive experience in performing comprehensive program developments and effective problem-solving. For our NextIO™ programs, we are committed to delivering the finalized program to our partners within about 1.5 years. The accurate timeline will be determined on a case-by-case basis. Here is a draft timeline for your quick glance.
 Fig.5 The timeline of Next-IOᵀᴹ programs.
Fig.5 The timeline of Next-IOᵀᴹ programs.
Your Success Is Our Priority
Creative Biolabs is looking for partners to help us gain a competitive edge in the field and maximize the impacts of the programs. With your support and collaboration, we are dedicated to developing novel cancer immunotherapies that can hope both parties to achieve greater success.
If you are interested in our program, please feel free to contact us for more details.



 Fig.1 Immune interactions involving antigen-presenting cells or tumor cells, T cells, and tumor microenvironment.1
Fig.1 Immune interactions involving antigen-presenting cells or tumor cells, T cells, and tumor microenvironment.1
 Fig.2 High level of VISTA is expressed in patients with endometrial and ovarian cancers.2
Fig.2 High level of VISTA is expressed in patients with endometrial and ovarian cancers.2
 Fig.3 Silencing VISTA expression in human cancer cells restores T cell proliferation and cytokine secretion.2
Fig.3 Silencing VISTA expression in human cancer cells restores T cell proliferation and cytokine secretion.2
 Fig.4 Anti-VISTA antibody treatment extends survival time in mice model.2
Fig.4 Anti-VISTA antibody treatment extends survival time in mice model.2

 Fig.5 The timeline of Next-IOᵀᴹ programs.
Fig.5 The timeline of Next-IOᵀᴹ programs.

 Download our brochure
Download our brochure