Ex Vivo Immune Reactivity Assessment Service
Creative Biolabs is the world's leading company in the discovery and development of novel cancer immunotherapies. With well-mature immune response assessment platforms and powerful operating software, we provide our worldwide customers with a convenient and efficient project service experience. Serving science and promoting science is always our goal.
Introduction to Immune Response Assessment
Currently, a cancer vaccine has been regarded as a novel cancer therapy strategy that introduces tumor antigen into the patient in a certain way to stimulate or enhance the patient's immune system and induce the body to produce long-term humoral and cellular immune responses, thus killing tumor cells. However, due to the difficulty in antigen preparation, low immunogenicity, and inability to permanently activate the immune system, the efficacy of cancer vaccines is still not satisfactory. As a result, as a top oncology research CRO company, Creative Biolabs has been pushing to refine and establish criteria for evaluating the therapeutic efficacy and immune response of tumor vaccines.
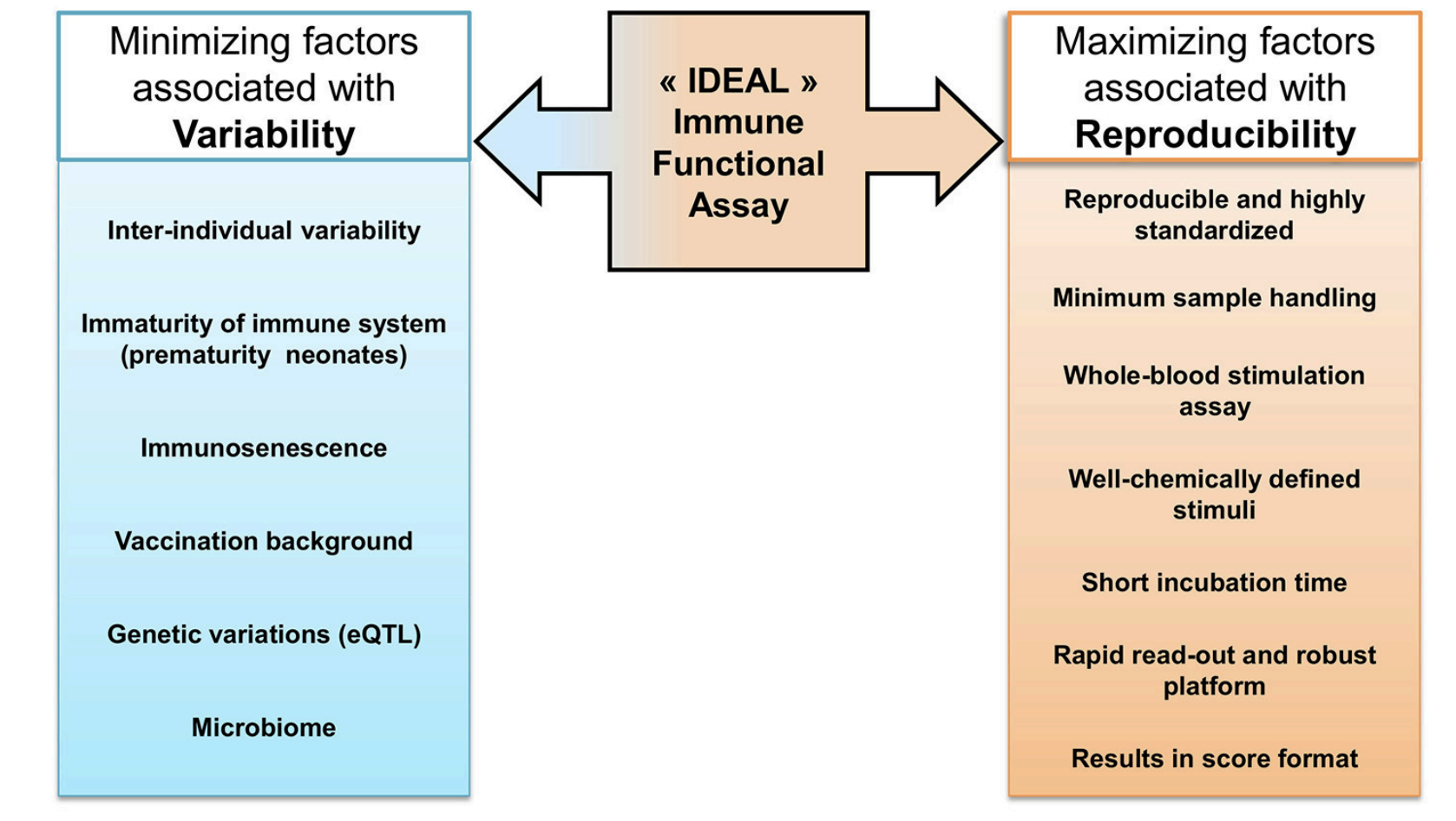 Fig.1 Immune Function Assay Protocol.1
Fig.1 Immune Function Assay Protocol.1
We combined traditional imaging systems, such as CT and MRI, with changes in tumor metabolic characteristics to determine the vaccine response. Meanwhile, dynamic real-time monitoring of various cancer markers, including markers existing in the tumor itself, markers related to the immune response in the microenvironment, and host-derived markers, will be integrated with the existing evaluation criteria of cancer immunotherapy. Moreover, by optimizing existing biostatistical analysis methods, we will promote the establishment of accurate immune response assessment criteria and expand the population of benefit.
Cheif Services
In Creative Biolabs, we have developed a personalized regimen for assessing immune responses based on various cancer antigens. In general, we will evaluate different adjuvants or preparation processes to further enhance the immunological efficacy of the vaccine. Furthermore, we will also assess the optimal dosage, treatment duration, and injection site regimen. Additionally, we will assist customers in screening and identifying suitable neoantigens and incorporating them into antigen-presenting cells (APCs) through specific methods or forming immune complexes to improve the immune responses of cancer vaccines. With our ongoing advancements in cancer neoantigen purification technology, deepening understanding of antigen presentation processes, and continuous optimization of tumor vaccine design and preparation techniques, we possess extensive experience in immune modeling/experimental design and immune response evaluation.
Besides, we have established a series of assays for evaluating the effectiveness of commonly used cancer vaccines, including but not limited to, in vitro Th17 differentiation assay, cytokine detection, cytotoxic T lymphocytes (CTL) activity detection, T cell activation marker detection, antibody titer detection, ADCC/CDC detection. The following is a list of some of our common detection solutions.
Table 1 Cytokine Detection Services
|
|
ELISA
|
ELISPOT
|
CBA
|
IFC
|
|
Measurements
|
1
|
2-10
|
>10
|
Multiple
|
|
Samples
|
Cell culture supernatant
|
Cells
|
Cell culture supernatant
|
Cells
|
|
Equipment
|
ELISA Analyzer
|
ELISPOT Reader
|
Flow cytometer
|
Flow cytometer
|
|
Advantages
|
Low cost; Absolute quantification of concentration, high repeatability
|
The cell frequency of secreting cytokines determination, high sensitivity
|
The Qualitative and relative quantitative detection; A variety of cytokines detection
|
Determination of the percentage of cells secreting cytokines
|
Table 2 CTL Activity Detection Services
|
|
Cell Viability Assay
|
Membrane Integrity Detection
|
Reporter Gene Detection
|
RTCA Detection
|
Cell Count Analysis
|
|
Measurements
|
Cell-Titer-GLo
|
LDH release
|
Reporter gene expression level
|
Unlabeled cells
|
Living cells/dead cells
|
|
Equipment
|
Luminescent microplate reader
|
Fluorescence Reader
|
Luminescent microplate reader
|
RTCA analyzer
|
Fluorescence imaging
|
|
Advantages
|
Endpoint method; Low cost
|
Endpoint method; Low cost
|
Endpoint method, Low cost, Low detection background, simple operation
|
Real-time monitoring, simple operation
|
Data visualization and rich data
|
Highlights
-
Innovative multi-omics research services
-
High throughput automated experiment and data analysis platform
-
Comprehensive measuring and testing ability
-
Efficient and cost-effective service chain
-
Fast turnaround time
Cheif Services
As an innovative technology service company, Creative Biolabs has a deep insight into customer needs, takes comprehensive innovation as its core value, and is committed to becoming a life science technology service leader driven by immunology research, making continuous breakthroughs in cutting-edge cancer immunotherapy technology research and development cooperation and transformation, and leading the new pattern of immune response assessment through innovation. If you are interested in our services, please feel free to contact us.
Reference
-
Albert-Vega, C.; et al. Immune functional assays, from custom to standardized tests for precision medicine. Frontiers in immunology, 2018, 9: 413410.
For Research Use Only | Not For Clinical Use


 Fig.1 Immune Function Assay Protocol.1
Fig.1 Immune Function Assay Protocol.1
 Download our brochure
Download our brochure