Next-IO™ LGR5 Therapeutic Monoclonal Antibody Program
About This Program
This program aims to develop LGR5 therapeutic monoclonal antibody for colorectal cancer immunotherapy.
Rationale for our program:
-
Colorectal cancer (CRC) is the third most common malignancy diagnosed globally and the fourth leading cause of cancer-related deaths worldwide. The burden is expected to increase by 60% at 2030.1. There is, therefore, an urgent need to develop new therapies to target CRC treatment.
-
A fundamental event in the early development of CRC is the dysregulation of the Wnt/β-catenin signaling pathway. Studies have shown that LGR5 (also known as GPR49) synergizes with Wnt receptors to enhance Wnt/β-catenin signaling, indicating the therapeutic merit of targeting LGR5 in CRC.
These findings suggest that our next-IO™ antibody targeting LGR5 is likely to revolutionize CRC therapy.
LGR5
LGR5 belongs to the class A rhodopsin-like GPCR superfamily. But now it has a well-identified ligand, R-Spondin (RSPO), which, when combined, works synergistically with Wnt receptors to enhance Wnt/β-catenin signaling. Figure 1 depicts the relationship between LGR5 and Wnt/β-catenin signaling.
Highlight Functions
-
Lgr5 has been shown to be up-regulated in various cancer tissues, such as basal cell carcinoma, hepatocellular carcinoma, colorectal tumors, and ovarian tumors.
-
Lgr5 has been shown to promote cancer cell migration, tumor formation and epithelial-mesenchymal transition in breast cancer cells by activating Wnt/β-catenin signaling.
-
It has been reported that there is a positive correlation between high expression of Lgr5 and short survival of patients.
-
Studies have further shown that Lgr5 regulates the malignant phenotype in patient-derived glioblastoma stem cell subsets, which may represent a potential predictor of glioblastoma.
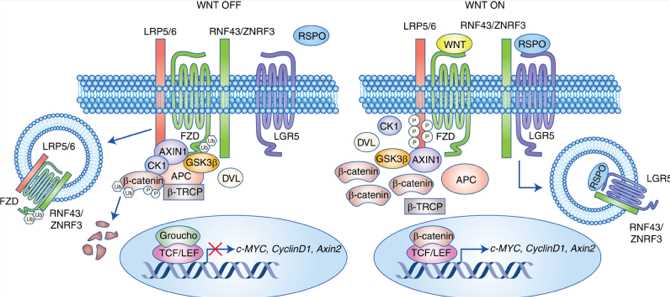 Fig.1 LGR5 promotes Wnt/β-catenin signaling. 1,3
Fig.1 LGR5 promotes Wnt/β-catenin signaling. 1,3
Published Data
-
Anti-LGR5 antibody and its ADC format inhibit tumor growth and recurrence in vivo.
 Fig.2 The antitumor effect of anti-LGR5 antibody. 2,3
Fig.2 The antitumor effect of anti-LGR5 antibody. 2,3
-
Inhibition of LGR5 with AZD8931 (AZD) significantly blocked EC CM-induced colorectal tumor growth.
These data support the rationale for the development of the first-in-class human LGR5 Abs, especially LGR5 antibody conjugate with an improved therapeutic index for the treatment of colorectal cancer.
Colorectal cancer
-
Colorectal cancer is the most common type of gastrointestinal cancer, and its incidence is increasing in developed countries.
-
The global cancer immunotherapy market is worth $58.1 billion in 2018. The expanding research of immunotherapy will become one of the important trends in the global colorectal cancer treatment market from 2019 to 2023.
-
Immunotherapy products such as immunological checkpoint inhibitors and vaccines are part of active research.
-
The global colorectal cancer treatment market research report predicts that the market will grow at a compound annual growth rate of more than 8% during the forecast period.
Ongoing Clinical Trials
Currently, only one therapeutic antibody against LGR5 is being investigated in clinical phase 1 studies. Our program still holds a broad market prospect in the IO field.
|
NCT ID
|
Status
|
Sponsor
|
Project
|
Phase
|
Update Time
|
|
NCT02726334
|
Terminated (Monotherapy arm completed. Combination arm did not proceed (sponsor decision).)
|
Bionomics Limited
|
A Phase I, Dose Escalation Study of an anti-LGR5 antibody in Patients With Metastatic Colorectal Cancer.
|
Phase 1
|
January 16, 2019
|
-
In an effort to optimally leverage LGR5-mediated immune response, our next-IO™ LGR5 targeted antibody program attempts to explore the optimal dual-targeted combination therapy by involving other immunomodulatory agents. We wish to work together with our partners to explore the discovery journey of the next generation of antibody drugs and achieve commercial success.
Program Management
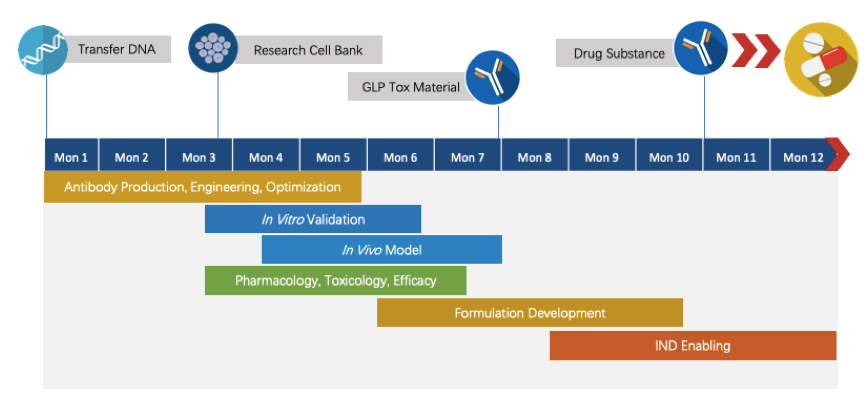
With extensive experience in the development of discovery and development of therapeutic antibodies, Creative Biolabs is committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage. Here is a draft timeline for your glance.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop LGR5 program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership together. For any partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners further their programs with more chances to succeed. Look forward to cooperating with you in the near future.
References
-
Morgan, R. G., E. Mortensson, and A. C. Williams. "Targeting LGR5 in Colorectal Cancer: therapeutic gold or too plastic?." British journal of cancer 118.11 (2018): 1410-1418.
-
Gong, Xing, et al. "LGR5-targeted antibody–drug conjugate eradicates gastrointestinal tumors and prevents recurrence." Molecular cancer therapeutics 15.7 (2016): 1580-1590.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use


 Fig.1 LGR5 promotes Wnt/β-catenin signaling. 1,3
Fig.1 LGR5 promotes Wnt/β-catenin signaling. 1,3
 Fig.2 The antitumor effect of anti-LGR5 antibody. 2,3
Fig.2 The antitumor effect of anti-LGR5 antibody. 2,3

 Download our brochure
Download our brochure