Regulatory T Cell Therapy Development Services
Regulatory T Cell (Tregs) Are Attractive Candidates for Immunotherapy
Accounting for 5-10% of CD4+ T cells, Tregs employ various methods to suppress the immune system and safeguard against autoimmune disorders. This suppression can occur through direct interactions, like engaging with CTLA-4, or indirectly by releasing cytokines. With their proven ability to prevent autoimmune diseases, Tregs hold great promise in promoting tolerance. Despite being a minority within the overall population of CD4+ T cells, Tregs are highly sought-after for their therapeutic potential in immunotherapy.
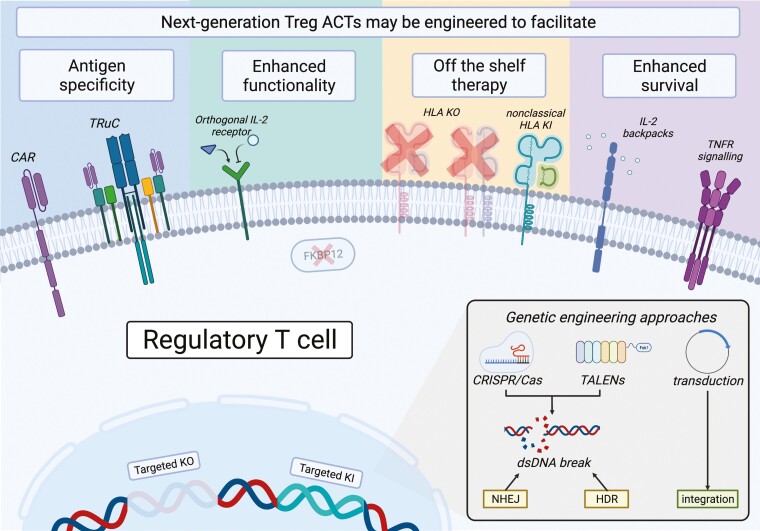 Fig.1 Emergent technologies present novel ways to engage Tregs.1
Fig.1 Emergent technologies present novel ways to engage Tregs.1
Next-generation Tregs Therapy Development Services
Antigen specificity, enhanced functionality, off-the-shelf therapy, and enhanced survival are the four advanced approaches to facilitate the development of Treg therapy by genome editing and bioengineering technologies.
Our team of experts is dedicated to supporting the development of Tregs therapy through a comprehensive range of services, including Treg isolation and expansion, Treg subpopulation expression profiling, genetic modification of Tregs using viral transduction, phenotypic and functional characterization, suppression assay, migration assay, and assessment of the effect of Treg on T cell transendothelial migration.
Treg Subpopulation Expression Profiling
This service involves analyzing the expression profile of Treg subpopulations. It provides insights into the specific markers and characteristics of different Treg subsets.
Treg Functional Profiling in Tumors
This service focuses on evaluating the functional properties of Tregs within tumor microenvironments. It helps assess the impact of Tregs on immune responses and tumor progression.
In Vitro Regulatory T Cell (Treg) Suppression Assay
This service enables the assessment of Treg-mediated suppression of immune responses in vitro. It helps understand the regulatory capabilities of Tregs and their potential implications in immune-related diseases.
Tregs Migration Assay
This service involves evaluating the migratory capacity of Tregs. It involves studying the movement of Tregs towards specific chemotactic cues, which can provide insights into the role of Tregs in immune cell trafficking.
Assess the Effect of Treg on T Cell Transendothelial Migration
This service focuses on understanding the impact of Tregs on the migration of T cells across endothelial barriers. It aids in elucidating the role of Tregs in modulating immune cell infiltration and their potential implications in autoimmune diseases and cancer.
Improving Treg specificity with chimeric antigen receptors (CAR) is a promising therapeutic approach for a variety of immune-mediated disorders. Our team consists of highly skilled scientists and experts with extensive experience in the field of immunology. With our CAR-Tregs development services, we aim to provide clients with a comprehensive and tailored approach to develop and optimize CAR-Tregs for their specific needs. We offer a range of services, including Treg isolation and expansion, CAR design and construction, CAR-Tregs characterization, and in vitro and in vivo efficacy studies.
Creative Biolabs offers comprehensive solutions for the development of Treg therapy. Should you require further information regarding this service, just get in touch with us. We eagerly anticipate working together with you and helping you achieve your objectives in the development of Tregs therapy.
Reference
-
McCallion, Oliver, et al. "Regulatory T-cell therapy approaches." Clinical and Experimental Immunology 211.2 (2023): 96-107. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use


 Fig.1 Emergent technologies present novel ways to engage Tregs.1
Fig.1 Emergent technologies present novel ways to engage Tregs.1
 Download our brochure
Download our brochure