Chimeric Antigen Receptor (CAR) T Cell Design and Manufacturing Service
Introduction
Structures of CAR-T
Mechanism of CAR-T-Mediated Tumor Death
CAR-T Precision and Safety Improvement
Services
Workflow of CAR-T Cell Design and Manufacturing
Why choose us?
Resources
Chimeric Antigen Receptor (CAR) T-cell therapy is a groundbreaking immunotherapy designed to re-engineer a patient's T-cells, enabling them to identify and destroy cancer cells. Creative Biolabs offers a comprehensive series of services in
cellular and gene therapies including T Cell Receptor (TCR) T cell therapy, CAR-T cell therapy, CAR-macrophage cell therapy,
and Chimeric Antigen Receptor (CAR) NK Cell Therapy. With a multidisciplinary team and flexible service models, Creative Biolabs provides tailored solutions to meet the unique requirements
of your research, ensuring seamless and integrated support throughout the therapeutic development process. We are willing to facilitate your studies and this service is only designed for research, not for clinical treatment.
Creative Biolabs offers comprehensive CAR-T cell design and manufacturing services, including neoantigen identification, scFv generation,
CAR design construction, and plasmid GLP production. Our end-to-end solutions cover the entire development process, from transfection and CAR-T preparation to
in vitro and
in vivo testing. We specialize in creating diverse CAR constructs, such as bispecific, inducible, and oxygen-sensing CARs, providing both GLP and GMP levels to support your research goals.
Our expert team ensures customized services, addressing all your CAR-T therapy development needs. If you want to know more about our detailed CAR-T cell design and manufacturing services, please click here for more information.
Introduction of CAR-T Cell Therapy
“We ensure careful consideration for the development of CAR-T therapy product to achieve efficient execution and regulatory approval.”
CAR-T cell therapy is an advanced form of immunotherapy designed to treat cancer. It involves engineering a patient's T-cells to express a receptor (CAR) that specifically recognizes antigens on tumor cells. This therapy redirects the body's immune system
to target and destroy cancer cells, showing substantial promise in hematological malignancies such as B-cell leukemia and lymphoma.
Creative Biolabs supports the development of various CAR generations, including bispecific, inducible, and synNotch CARs, to meet evolving immunotherapy needs, focusing on providing tailored solutions that address both hematological and solid tumors.
CAR-T Structures
Chimeric antigen receptors (CARs) are engineered receptor constructs consisting of an extracellular single-chain variable fragment (scFv) derived from an antibody specific to a tumor neoantigen. This scFv is linked via a hinge or spacer peptide
to a transmembrane domain, further connecting to intracellular signaling domains of the T-cell receptor. Combining the specificity of an antibody with the cytotoxic and memory functions of T cells, CAR-T cell therapy shows powerful and promising therapeutic
functions for cancer treatment in high affinity without MHC dependent requirement.
-T-Cell-Design-and-Manufacturing-Service-2.png) Fig.
1 The Structure and Mechanism of Action of CAR-T Cells.1,4
Fig.
1 The Structure and Mechanism of Action of CAR-T Cells.1,4
The Mechanism of CAR-T-Mediated Tumor Death
CAR-T cells destroy cancer cells through several mechanisms. One primary method involves releasing cytotoxic granules containing perforin and granzymes, which create pores in tumor cell membranes and induce apoptosis via intracellular death
pathways. Another pathway is the Fas and Fas ligand (FasL) system, which activates caspases to trigger cell death. Additionally, CAR-T cells produce cytokines that modify the tumor microenvironment, enhancing immune responses and amplifying anti-tumor
activity. These mechanisms collectively contribute to the potent anti-cancer effects of CAR-T therapy.
-T-Cell-Design-and-Manufacturing-Service-3.jpg) Fig.
2 CAR T Cells Mediate Tumor Killing via Three Axes2,4
Fig.
2 CAR T Cells Mediate Tumor Killing via Three Axes2,4
Enhancing CAR-T Precision and Safety via Combinatorial Antigen Sensing
There are three different strategies for CAR-T cells to enhance specificity and reduce off-target effects through conditional combinatorial antigen detection. The "AND" gates activate CAR-T cells only when two antigens are present, using a synthetic Notch
receptor (synNotch) to detect antigen A, which triggers the expression of a CAR targeting antigen B. Differing from "AND" gates strategy, "OR" gates activate CAR-T cells when either of two antigens is recognized, preventing immune evasion by tumor
cells that downregulate one antigen. For instance, bispecific CARs use "OR" gates to recognize two antigens simultaneously, reducing tumor escape risk. By linking scFv domains in tandem, they optimize targeting efficiency, increasing therapeutic potential
while minimizing antigen-loss and improving cancer cell detection. "AND-NOT" gates introduce inhibitory receptors (iCARs), co-expressing an antigen-binding domain for healthy tissues linked to inhibitory signaling domains like PD-1 or CTLA-4 and deactivating
CAR-T cells in response to healthy tissue antigens, reducing off-target toxicity. These strategies collectively improve CAR-T precision and safety.
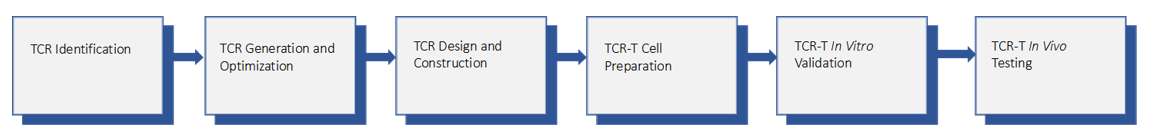
CAR-T Cell Design and Manufacturing Services
“We are dedicated to accelerating your TCR-T therapy product through a series of innovative and advanced platforms.”
CAR-T cell therapy has been considered the most significant therapeutic advance in a generation. To develop CAR-T based approaches, Creative Biolabs offers an end-to-end integrated solution covering all steps prior to clinical trials. You can start at
any module and request a flexible integration of different modules to fit your projects. Both engineering services for αβ T cell and γδ T cell derived from both abnormal and normal populations can be achieved.
-T-Cell-Design-and-Manufacturing-Service-4.jpg) Fig.
3 Process of CAR-engineered T cell therapy.3,4
Fig.
3 Process of CAR-engineered T cell therapy.3,4
The Process of CAR-T Cell Design and Manufacturing Available on Creative Biolabs
Creative Biolabs provides an extensive range of CAR-T cell design and manufacturing services, from neoantigen identification and scFv generation to CAR design and plasmid GLP production. Our solutions encompass the full development process, including
transfection, CAR-T preparation, and in vitro/in vivo testing. We excel in engineering various CAR constructs, such as bispecific, inducible, and oxygen-sensing CARs, and offer both GLP and GMP support. With a highly skilled
team, we deliver tailored solutions to support all aspects of CAR-T therapy development.
With multiple gene and protein analysis technologies, we offer unique de novo neoantigen discovery, identification, and selection to get a suitable CAR-T therapy target with high potency and low toxicity.
Aided by our in-house antibody database and various types of libraries including hybridomas, mouse, rabbit and human, plus scFv sequencing and affinity measurement platforms, we are dedicated to generating the most optimized scFv with high affinity and
good properties.
We are capable of designing and constructing different generations of CARs, also various types of engineered CARs are available at Creative Biolabs, such as suicide CAR, inducible CAR, enhanced CAR, bispecific CAR, synNotch CAR, masked CAR, oxygen-sensing
CAR, etc.
With various transfection technologies including electroporation, as well as cell transduction through adenovirus, lentivirus retroviral, etc., we offer CAR gene packaging and delivery services for CAR-T cell generation. To facilitate your drug development,
both GLP and GMP levels can be achieved to fit your purpose of the project.
For generated CAR-T products, a wide range of in vitro assays are available to evaluate the efficiency of CAR-T products, including CAR expression validation, CAR-T cell proliferation, cytokine release assay, cytotoxicity assay, etc.
Once in vitro assays are completed, a series of in vivo testing systems will be performed to evaluate the CAR-T product, including animal models, biodistribution studies, efficacy testing, toxicology assessment, and other GLP
compliant assays.
Highlights of Our CAR-T Design and Manufacturing Services
“Let Creative Biolabs support your CAR-T therapy breakthrough, feel free to speak with our CAR-T cell therapy experts.”
With the commitment to being your best CAR-T therapy partner, Creative Biolabs has established the utmost efficient integrated solutions to innovate and accelerate your drug developments for various types of cancers, especially hematologic malignancies.
We are willing to provide you with customized CAR-T design and manufacturing services suitable for your multiple requirements, the reasons why we deserve to be chosen including but not limited to:
-
Expertise: with in-depth knowledge and experience in cancer therapies, we provide cutting-edge CAR-T solutions.
-
Collaboration: our multidisciplinary team is composed of clinicians, hematologists/oncologists, pulmonologists and cardiologists.
-
Customization: we tailor its CAR-T services to meet the specific requirements of each project, from neoantigen discovery to clinical validation.
-
Other relevant options: we also provide drug development programs including antibodies, vaccines, small molecules, and others designed to stimulate the immune system to eliminate various types of cancer. Our experts will accommodate the
characteristics of your project and design the most appropriate treatment option for you.
Come and let Creative Biolabs support your CAR-T therapy breakthrough, please feel free to contact us to get more information and a quote for any CAR-T therapy module.
References
-
Chen, Yi-Ju, Bams Abila, and Yasser Mostafa Kamel. "CAR-T: what is next?" Cancers 15.3 (2023): 663.
-
Benmebarek, Mohamed-Reda, et al. "Killing mechanisms of chimeric antigen receptor (CAR) T cells." International journal of molecular sciences 20.6 (2019): 1283.
-
Sadeqi Nezhad, Muhammad, et al. "Chimeric antigen receptor based therapy as a potential approach in autoimmune diseases: how close are we to the treatment?" Frontiers in immunology 11 (2020): 603237.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use



-T-Cell-Design-and-Manufacturing-Service-2.png) Fig.
1 The Structure and Mechanism of Action of CAR-T Cells.1,4
Fig.
1 The Structure and Mechanism of Action of CAR-T Cells.1,4
-T-Cell-Design-and-Manufacturing-Service-3.jpg) Fig.
2 CAR T Cells Mediate Tumor Killing via Three Axes2,4
Fig.
2 CAR T Cells Mediate Tumor Killing via Three Axes2,4
-T-Cell-Design-and-Manufacturing-Service-4.jpg) Fig.
3 Process of CAR-engineered T cell therapy.3,4
Fig.
3 Process of CAR-engineered T cell therapy.3,4
 Download our brochure
Download our brochure