TCR-T and CAR-T/NK Cytotoxicity Assay
T/NK-cell mediated cytotoxicity assay can be used to assess small molecule drug candidates, T cell therapy or antibody-based targeted tumor cell killing, for example, immunotherapy strategies that seek to modulate immune responses by promoting T/NK cell recognition of tumor antigens.
Creative Biolabs provides T/NK cell cytotoxicity assays through advanced instruments. Our services include long-term cytotoxicity assays to reveal T/NK cell killer-mediated cytotoxicity in cell culture. We can use a wide range of cancer and healthy cell lines, for example, using various types of induced pluripotent stem cells (iPSCs) such as the heart, liver, and brain. We provide the assessment of the cytotoxicity/safety of a panel of related healthy cell lines for a wide range of cells and compounds, including but not limited to TCR, CAR, potential cytotoxic agents, and medical drugs.
As a complement to the cytotoxicity analysis, we performed flow cytometry with intracellular and extracellular staining, allowing us to study the cytotoxicity of direct T/NK cells or other cells in parallel and to study the induction of cells or compounds after co-culture at single cell resolution.
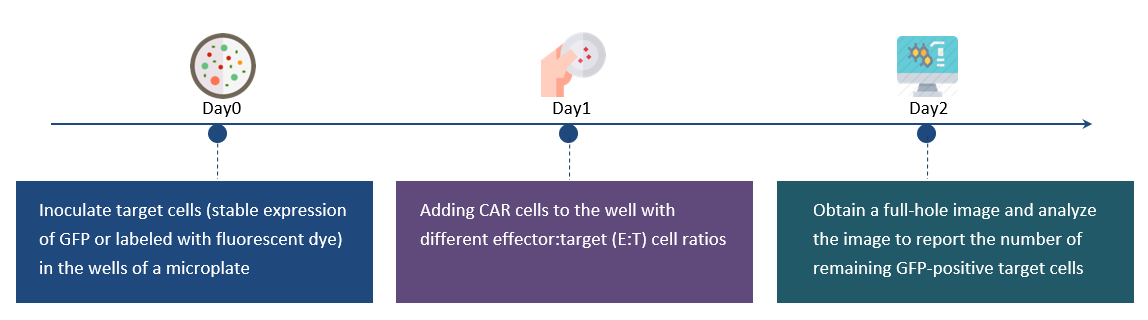
Workflow for Cytotoxicity Assay
Key Benefits
-
The same plate is scanned at multiple time points to directly monitor the killing of target cells
-
Full-bore imaging and analysis of the whole 96-well plate in 15 minutes
-
No trypsinization of cells is required, and then a longer flow cytometry protocol is performed. Creative Biolabs directly calculates and reports each target cell remaining in each well
For more details, please feel free to contact us for project quotations and more detailed information.
For Research Use Only | Not For Clinical Use




 Download our brochure
Download our brochure