Toxicity Assessment Service
Based on our advanced toxicity assessment service and powerful technology, Creative Biolabs provides a complete range portfolio of assays for toxicity measurement by biomarkers. To meet the kind of service you need, our highly skilled scientists are ready to get started on your projects.
Introduction of Cancer Vaccine Toxicity Assessment
Cancer vaccine is a type of adaptive immunotherapy by engineering cancer cells with self-destruction strategies. It could achieve good effectiveness, by inducing extensive local lymphangiogenesis and promoting stronger T-cell activation. As a special kind of pharmaceutical, cancer vaccines not only should be submitted to strict non-clinical safety evaluation but also need to be assessed for potential toxicities, which part has been included in guidelines, such as ICH S6. Fig.1 showed the drug-induced hepatocyte damage with complex, multivariant host response, which could be detected by measurement of serum biomarkers. The biomarker measurement services we provide include but are not limited to alanine aminotransferase, alkaline phosphatase, amylase, calcium, glucose, cholesterol, electrolyte, creatine kinase, total bilirubin, and total protein.
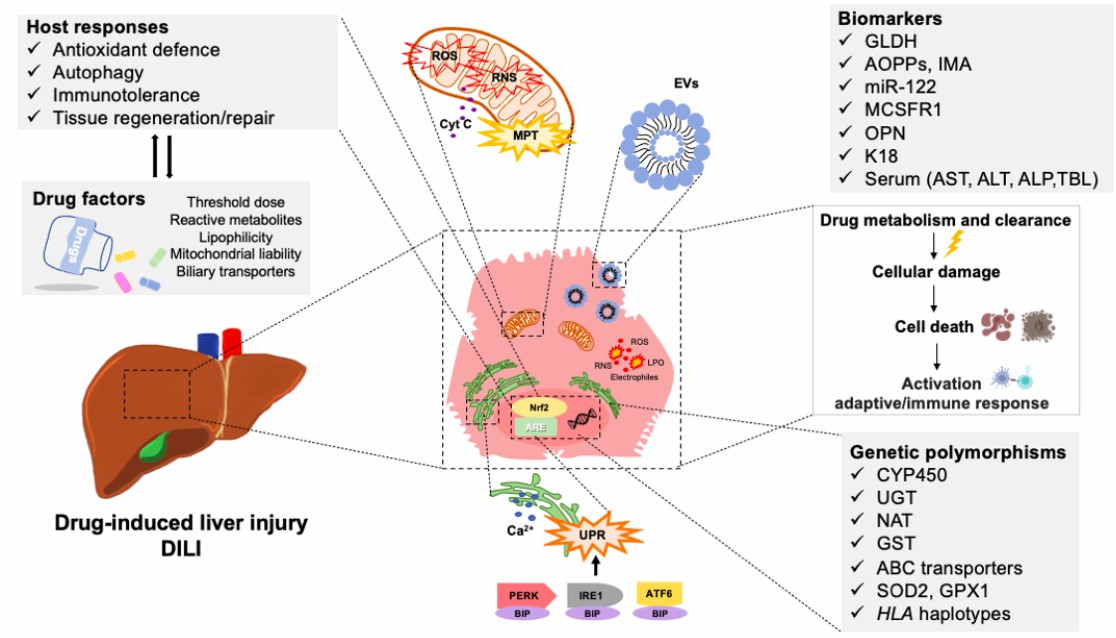 Fig.1 Mechanisms and biomarkers of drug-induced liver injury. (Villanueva-Paz, et al., 2021)
Fig.1 Mechanisms and biomarkers of drug-induced liver injury. (Villanueva-Paz, et al., 2021)
Toxicity Assessment Services at Creative Biolabs
An inescapable problem of cancer immunotherapy must be addressed is its unique set of immune-related adverse events, which may be potentially life-threatening. Thus, it is essential to identify these toxic events early and help with the treatment appropriately. The toxicity effects of cancer patients treated with Self-destruction immunotherapy are often characterized by rapidly and dramatically elevated high levels of serum or urinary biomarkers, such as alanine aminotransferase, alkaline phosphatase, electrolyte, and creatine kinase.
Measurement of these biomarkers with our toxicity assessment services could help researchers evaluate and minimize the lethal risks of uncontrolled adverse events, like a liver injury. Our toxicity assessment service could also contribute to the acquirement of good candidates for cancer immunotherapy and the dose optimization to insure the candidates could work well and achieve efficient anti-tumor immune responses in the early stage of cancer vaccine or drug development. Ultimately, Our toxicity assessment service is a basic and advisable tool for the valuation of the immune effect of cancer immunotherapy.
Compared with in vivo immune reactivity assessment, our monitoring platform of the immune responses and toxic effects has many advantages including lower risk, higher efficiency, and fewer technical difficulties. With decades of in-depth study of this area, we are the one most potent professional technical developers. We will bridge the gap between your sample materials and a well-rounded assessment and analysis result of your specific immune strategies.
Toxicity Assessment by Quantification of Alanine Aminotransferase (ALT)
Creative Biolabs offers advanced custom analytic services for the quantification of ALT with the combination of our software and multiplex analyzers including spectrophotometric, chemiluminescence, fluorescence, and UV absorbance.
Toxicity Assessment by Quantification of Alkaline Phosphatase (ALP)
Toxicity assessment by quantification of ALP, could help detect liver diseases like hepatitis, cirrhosis, tumor, abscess, granulomas, or amyloidosis. ALP detection has been researched well by scientists of Creative Biolabs.
Toxicity Assessment by Quantification of Amylase
Amylase is the key enzyme involved in the carbohydrate digestion process, its inhibitors have been regarded as new drugs to treat T2DM. Creative Biolabs is one potent professional technical developer on the quantification of Amylase.
Toxicity Assessment by Quantification of Calcium
Calcium has diverse important physiological functions and is a sensitive and non-invasive biomarker. As an end-to-end company, Creative Biolabs offers high-quality analytical development and qualification service for calcium.
Toxicity Assessment by Quantification of Glucose
Glucose is the most abundant and important monosaccharide as a source of energy.
Considering the importance of glucose toxicity research in pharmacological development, glucose measurement has been researched well by scientists of Creative Biolabs.
Toxicity Assessment by Quantification of Cholesterol
The occurrence and distribution in the body fluid of different cholesterol types have been widely used as important biochemical parameters for toxicity assessment, like atherosclerosis. Cholesterol measurement has been researched well by scientists of Creative Biolabs.
Toxicity Assessment by Quantification of Electrolyte
Electrolytes are minerals that have many functions in the body. Measurement service of electrolyte levels in the treatment is a fundamental requirement to identify the potential risks of the progression to chronic kidney disease.
Toxicity Assessment by Quantification of Creatine Kinase
Creatine kinase (CK) is a fundamental enzyme regulator of cellular energy homeostasis. Through CK measurement, Creative Biolabs could help timely monitor possible toxic effects caused by the metabolism of candidate drugs or vaccines.
Toxicity Assessment by Quantification of Total Bilirubin
Bilirubin is a daily generated endogenous compound as the ultimate breakdown product of hemoglobin. Measurement of total bilirubin levels is necessary for dose optimization to manage bilirubin abnormalities and prevent the occurrence of toxicities.
Toxicity Assessment by Quantification of Total Protein
Proteins are essential for overall body health. Creative Biolabs is one most potent professional technical developers of the total protein measurement for toxicity assessment.
Our experienced experts at Creative Biolabs will complete your project of toxicity assessment service you needed. If you are interested in this, please feel free to contact us directly.
Reference
-
Villanueva-Paz, M; et al. Oxidative stress in drug-induced liver injury (DILI): from mechanisms to biomarkers for use in clinical practice. Antioxidants. 2021, 10(3): 390.
For Research Use Only | Not For Clinical Use


 Fig.1 Mechanisms and biomarkers of drug-induced liver injury. (Villanueva-Paz, et al., 2021)
Fig.1 Mechanisms and biomarkers of drug-induced liver injury. (Villanueva-Paz, et al., 2021)
 Download our brochure
Download our brochure