Virus GLP Production
 According to the latest cell gene therapy research, Creative Biolabs provides the GLP virus production platform for
cancer treatment, including the TCR design and CAR design process systems. As a leading market
forefront cell
therapy company, we design a huge carrier construction of laboratory with strict operation procedures, and we are
devoted to offering the most convenient and efficient technical services to help you achieve your scientific
research goal.
According to the latest cell gene therapy research, Creative Biolabs provides the GLP virus production platform for
cancer treatment, including the TCR design and CAR design process systems. As a leading market
forefront cell
therapy company, we design a huge carrier construction of laboratory with strict operation procedures, and we are
devoted to offering the most convenient and efficient technical services to help you achieve your scientific
research goal.
GLP Standard Laboratory Platform
In order to ensure the safety of your virus products, we’ve made great efforts to establish GLP standard laboratory,
and are capable of providing production services and QC tests through a standardization process. Each step has a
strict SOP specification operating system, including:
-
Raw materials
-
Manufacturing process
-
Biological stability and physical and chemical properties of the quality inspection and analysis
-
The final product of the reasonable standard packaging and transportation
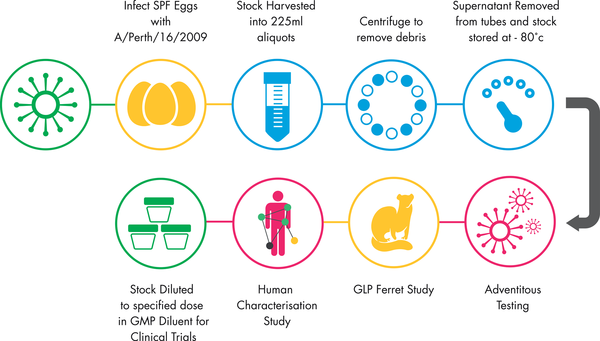 Fig.1 Summary of Production of the GMP Challenge Stock.
Fig.1 Summary of Production of the GMP Challenge Stock.
Virus GLP Vector Platform
The vector production facility produces lentivirus and retrovirus vectors in a sterile room, which is compliant with
FDA's Current Good Laboratory Practice (GLP) and is used to produce viral vectors that support early clinical
trials.
Creative Biolabs has many viral vectors that can be transformed into a conditionally replicative oncolytic virus
with oncolytic activity by molecular biology, including:
√ Adenovirus vector
√ Retrovirus vector
√ Herpes simplex virus vector
√ Vaccinia virus vector
Highlights
-
Able to offer different types of GLP virus vector to meet your experimental requirements,
-
Reasonable distribution of animal rooms and supporting facilities, and strict temperature control,
-
The high-quality staff has the knowledge structure, work experience and professional proficiency required to
complete the research work,
-
Good laboratory facilities and equipment ensure the normal, smooth, timely and high-quality completion of
research work,
-
Establish a sound organization and management system to promote the successful completion of virus production.
Creative Biolabs has established advanced and first-class facilities, such as molecular biology laboratory,
the
cytology laboratory and the virology laboratory, with larger production scale and more diversified production
capacity, which enables a variety of suspension and adhesion production platforms to be created. If there is any
interest in our novel oncolytic treatment services, just contact us and obtain more information.
Reference
-
Fullen D J; et al. Accelerating influenza research: vaccines, antivirals, immunomodulators and monoclonal
antibodies. The manufacture of a new wild-type h3n2 virus for the human viral challenge model[J]. PLOS ONE, 2016,
11(6): e0157211.
For Research Use Only | Not For Clinical Use



 Fig.1 Summary of Production of the GMP Challenge Stock.
Fig.1 Summary of Production of the GMP Challenge Stock.
 Download our brochure
Download our brochure