Pilot studies have suggested that glycosylation modification accounts for more than 50% of all PTM (post-translational modifications) in drug discovery. Meanwhile, the results for glycosylation analysis have been also used in a wide variety of therapeutic protein drugs (such as recombinant protein drugs, antibody drugs) production. Since the approval of the first antibody-drug OKT3 in 1986, the FDA has approved approximately 63 antibody drugs and 14 fusion protein drugs, as well as many other protein drugs (clotting factors, recombinases, etc.). Most of these drugs have glycosylation modifications that have dramatically altered the treatment of many diseases. However, glycosylation modification may the change of half-life, safety and effectiveness of candidate drugs in clinical use. Creative Biolabs focuses on the glycosylation analysis of various drugs, especially for antibodies, to improve their efficacy and provide you with advanced technology and services.
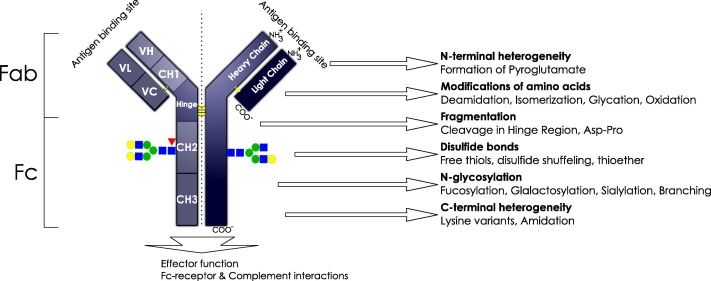 Fig.1 Schematic structure of an IgG1 molecule. (Higel, 2016)
Fig.1 Schematic structure of an IgG1 molecule. (Higel, 2016)
Glycosylation modification is influenced by many factors, such as cell lines, culture conditions, purification processes, and the like. Many glycosylation analysis techniques have been developed in Creative Biolabs, to help obtain a deeper understanding of the glycosylation process and effects, as well as to better control the glycosylation modification process. Currently, the glycosylated form which can provide by us is mainly N-glycosylation, and the monosaccharides involved are mainly: glucose, galactose, mannose, N-acetylglucosamine, N-acetylgalactosamine, fucose and sialic acid (NANA, NGNA).
ESI (electrospray ionization) source combined with high-resolution mass spectrometry is a common means of detecting protein molecular weight in our company. Taking monoclonal antibodies as an example, the intact molecular weight is close to 150 kDa, and a plurality of charges (about 40-70) can be carried in the ESI source so that the mass-to-charge ratio (m/z) of the monoclonal antibody ranges from 2500 to 3500. In this m/z region, high-resolution mass spectrometry such as TOF and Orbitrap can distinguish different glycoforms of monoclonal antibodies.
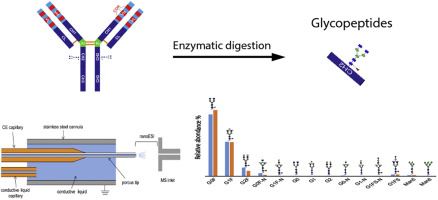 Fig.2 Graphical abstract of monoclonal antibody N-glycosylation profiling. (Giorgetti, 2018)
Fig.2 Graphical abstract of monoclonal antibody N-glycosylation profiling. (Giorgetti, 2018)
The rapid development of antibody drugs has promoted the industry's increasing emphasis on the modification process, analysis, control, and SAR relationship of glycosylation, and its complexity requires a more macroscopic and holistic perspective. Creative Biolabs has been committed to the manufacturability Assessment and optimization services for many years, from focusing on analysis to control, from optimizing stable cell culture processes to glycosylation, from formal analysis to SAR analysis, such as similar drug glycosylation comparability studies, glycosylation modification of subtype/glycosylation modifications in iterative antibody drugs or PD-1/PD-L1 antibody drugs. If you are interested in our glycosylation assay, please contact us and we will happy to help you.
References
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.