Oncolytic Viruses in Colorectal Cancer Treatment
Introduction of Colorectal Cancer (CRC)
CRC is the third most common tumor type in the world with high mortality. Approximately 50% of patients will develop liver metastases during the disease. Of all patients with liver metastases, only 10-20% are resectable. The main treatment of CRC is surgical resection systemic chemotherapy, and new biological agents. If resection is not effective, systemic chemotherapy is usually administered. Fluorouracil, irinotecan (FOLFIRI), leucovorin, infusional fluorouracil, oxaliplatin (FOLFOX), leucovorin, or capecitabine plus oxaliplatin are used in the first- and second-line treatments include. The standard chemotherapy combining with targeted drugs, such as bevacizumab, which is considered effective regarding overall survival, is also applied in the second-line treatment. Therapies for CRC are not considered curative. More efficient methods with satisfying curative effects and fewer side effects are highly needed.
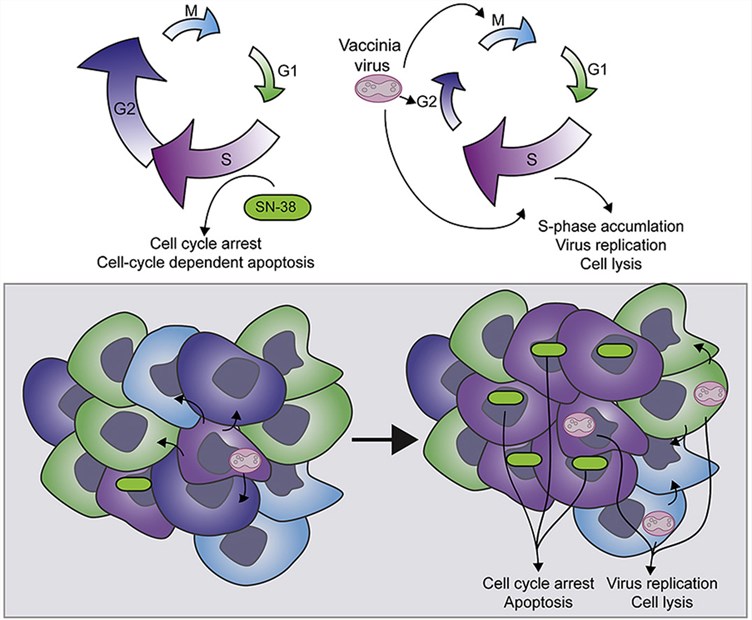 Fig.1 Vaccinia virus synergizes with SN-38 (active metabolite of irinotecan) in CRC cell lines. (Ottolino-Perry, 2015)
Fig.1 Vaccinia virus synergizes with SN-38 (active metabolite of irinotecan) in CRC cell lines. (Ottolino-Perry, 2015)
Oncolytic Viruses for the Treatment of Colorectal Cancer
- ONYX-015
- ICOVIR-5
- Western Reserve strain
- Pexa-Vec
Oncolytic virotherapy is a more recently emerging treatment option. ONYX-015 was a replication-competent E1B-deleted adenovirus, which selectively replicates in tumor cells. In a phase II study, ONYX-015 was assessed in 18 patients with treatment-refractory colorectal cancer. In 36% of patients, viral DNA was detected by PCR in plasma 72h after the last treatment. Immunohistochemistry (IHC) and PCR were performed in the spleen and normal liver of one patient who died of disease progression and the results indicated that viruses were with very low levels in tumor tissue.
ICOVIR-5 is a modified oncolytic adenovirus that has a deletion of the E1A region and has an RGD sequence inserted at the HI loop of the fiber knob to target integrins and assist in tumor cell entry. 12 advanced cutaneous and uveal melanoma patients were treated with a single dose of ICOVIR-5 at increasing doses. The results showed that 2 of 3 patients at the higher doses had virus detected by qPCR assay. Across all these studies, adverse events were largely low-grade constitutional symptoms and no objective clinical responses were seen.
A phase I study of a Western Reserve strain of vaccinia virus in 11 patients with advanced CRC was treated with a single IV dose in a dose-escalation design. The virus was detected in blood on days 3 and 8 while two patients had virus found in tumor tissue by PCR analysis.
Pexa-Vec is a thymidine kinase gene-deficient oncolytic vaccinia virus encoding GM-CSF. This oncolytic virus was assessed in 15 patients with CRC with increasing doses. In plasma 2 h after cycle 1 and 30 min after cycle 4, infectious viral titers were detected, as well as low doses in throat swabs 5-8 days after treatment.
Combination of Oncolytic Viruses with Immune Checkpoint Inhibitors in CRC Treatment
Immune-activation is an important way of determining the anti-tumor efficacy of oncolytic viruses. However, tumors often suppress the anti-tumor immune response, especially through the checkpoint axis. Therefore, it seems logical that the combination of the oncolytic virus with checkpoint inhibitors would result in the more effective treatment of cancer. Inhibitors of immune checkpoint protein programmed death 1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA4), have been shown to exert effective anti-tumor activity against a variety of malignancies in preclinical and clinical studies.
A combination of intra-tumoral injections of the oncolytic virus and systemically delivered anti-CTLA4 was found to be superior in controlling both injected and un-injected tumors compared to treatment with either alone. Another study demonstrated that the combination of an oncolytic vaccinia virus with a CTLA4 inhibitor enhances anti-tumor response in mouse models of colon cancer. These pre-clinical studies have offered a strong rationale for testing the combination of oncolytic viruses with checkpoint inhibitors in clinical settings. Indeed, a phase I clinical trial is ongoing to evaluate the combination of enadenotucirev (a chimeric adenovirus) with anti-PD-1 antibody (nivolumab) in CRC patients.
Based on our cut-edging OncoVirapy™ platform, Creative Biolabs offers a one-stop solution of oncolytic virus construction and oncolytic virus engineering. We will keep optimizing and expanding our services in cancer immunotherapy to meet the demand of next generation of cancer therapy.
References
- Chaurasiya, S.; Warner, S. Viroimmunotherapy for colorectal cancer: clinical studies. Biomedicines. 2017, 5(1):11.
- Ottolino-Perry, K.; et al. Oncolytic vaccinia virus synergizes with irinotecan in colorectal cancer. Molecular oncology. 2015, 9(8):1539-52.
