Oncorine
Oncorine is the first oncolytic adenovirus agent approved for the treatment of cancers. As a world-leading services provider, Creative Biolabs is always dedicated to providing a full range of oncolytic viral products and fast services for our worldwide customers with years of experience and high-end technologies.
Background of Oncorine
Conventional cancer therapies, such as chemotherapy, radiotherapy, and surgery, are primary approaches for current cancer treatment, which show limitations like high toxicity and non-targeting. Cancer immunotherapy based on the monoclonal antibody, immune checkpoint, and other immune components has achieved remarkable progress in cancer treatment over the past decades. But, the most puzzling cancer treatment problem for doctors and scientists is that almost all cancers can escape from the immune surveillance and immune killing. Exactly, cancer immunotherapy/gene therapy based on oncolytic virus emerges as an encouraging alternative for cancer treatment.
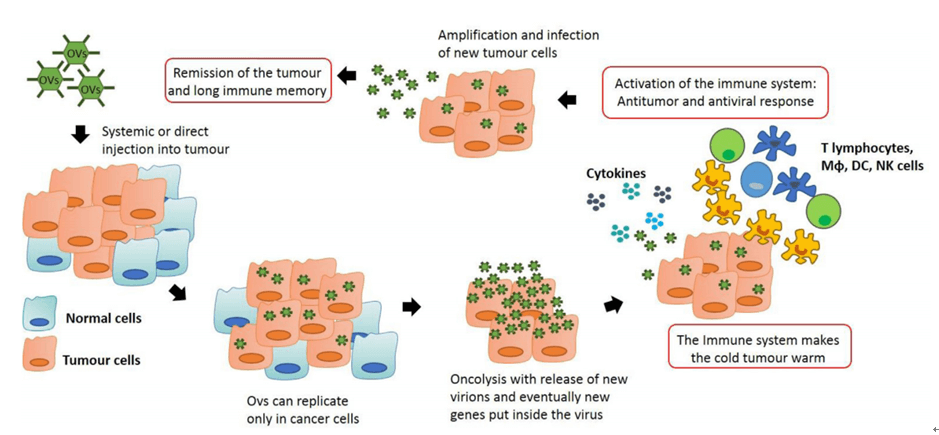 Fig.1 Anti-tumor immunity by oncolytic virus therapy. (Marelli, 2018)
Fig.1 Anti-tumor immunity by oncolytic virus therapy. (Marelli, 2018)
What is Oncorine?
Oncolytic viruses, a new class of therapeutic agents, are engineered viruses with abilities of both directly killing the tumor cells and stimulating host anti-tumor immune system responses. These oncolytic viruses can be adenovirus, autonomous parvoviruses, vaccinia virus, vesicular stomatitis virus, etc. On the one hand, oncolytic viruses can replicate in cancer cells but not in normal cells, causing lysis of the tumor mass. On the other hand, the lysed tumor cells release more infectious virus particles to infect and destroy the remaining tumor. They also release tumor-associated antigens into the microenvironment to stimulate the immune response.
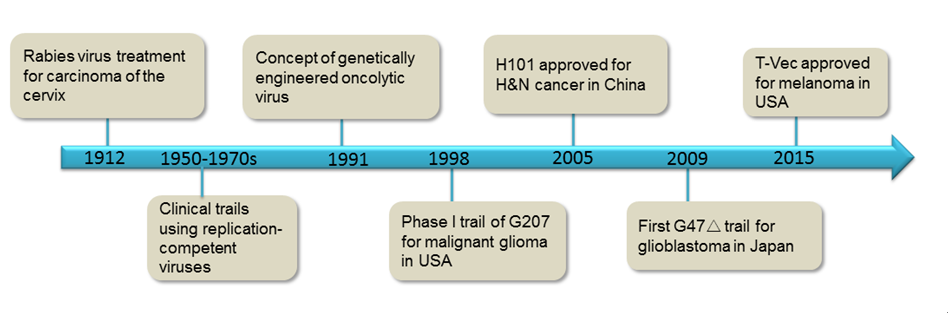 Fig.2 Milestones of oncolytic virus therapy development. (Fukuhara, 2016)
Fig.2 Milestones of oncolytic virus therapy development. (Fukuhara, 2016)
Among these oncolytic viruses, adenovirus is the first as well as the most extensively tested vector in the clinical trials. Oncorine, also referred to as Recombinant Human Adenovirus Type 5 Injection, was the first approved oncolytic virus by the Chinese State Food and Drug Administration (CFDA) for the cancer treatment in November 2005. Oncorine is a genetically modified adenovirus named H101 (E1B-deletion) developed by Shanghai Sunway Biotech in China, which is used in conjunction with chemotherapy for the treatment of nasopharyngeal carcinoma and head and neck cancer. The approval and successful application of Oncorine greatly promote the development of cancer immunotherapy based on the oncolytic virus. Numbers of oncolytic-viral biologic agents, including oncolytic adenoviruses, oncolytic herpes simplex viruses, vaccinia virus, vesicular stomatitis virus, measles virus, are in clinical trials currently for cancer therapy.
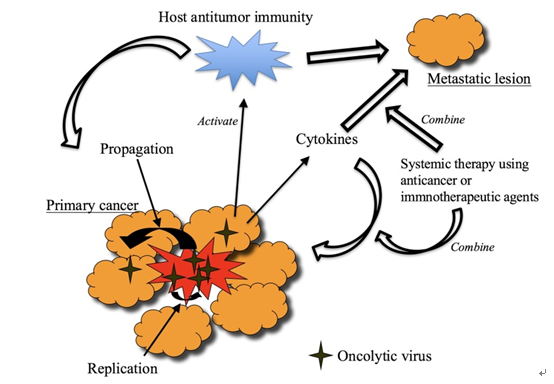 Fig.3 Mechanisms of action of oncolytic virus therapy. (Fukuhara, 2016)
Fig.3 Mechanisms of action of oncolytic virus therapy. (Fukuhara, 2016)
Start Your Oncolytic Virus Projects, Choose Creative Biolabs!
- Advanced and special OncoVirapy™ platform
- A number of engineered oncolytic viruses serotype products are available
- Highly experienced scientists and staff specialized in oncolytic virus-based gene therapy
- Highly customized service according to different research demands
As a biotechnology company keeping pace with times, Creative Biolabs has invested numerous energy and efforts in the development of oncolytic virus-based gene therapy. We now provide comprehensive engineering, construction, validation, preclinical research services about the oncolytic virus for global customers. Please feel free to contact us and our experienced technicians will give you the most detailed answers to your questions.
References:
- Marelli, G.; et al. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword Against Cancer. Frontiers in Immunology. 2018, 9: 866.
- Fukuhara, H.; et al. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Science. 2016, 107(10): 1373-1379.
