Rhodopsin (also known as visual purple), encoded by RHO gene, is a light-sensitive receptor protein involved in visual phototransduction. It was discovered by Franz Christian Boll in 1876 and was named after ancient Greek ῥόδον (rhódon) for "rose", due to its pinkish color, and ὄψις (ópsis) for "sight". Rhodopsin is a biological pigment discovered from the rods of the retina. It is also a G-protein-coupled receptor (GPCR). Rhodopsin is significantly sensitive to light, helping vision in low-light conditions. When exposed to light, rhodopsin immediately photobleaches. In humans, rhodopsin is regenerated fully in about 30 minutes.
| Basic Information of RHO | |
| Protein Name | Rhodopsin |
| Gene Name | RHO |
| Aliases | Opsin-2 |
| Organism | Homo sapiens (Human) |
| UniProt ID | P08100 |
| Transmembrane Times | 7 |
| Length (aa) | 348 |
| Sequence | MNGTEGPNFYVPFSNATGVVRSPFEYPQYYLAEPWQFSMLAAYMFLLIVLGFPINFLTLYVTVQHKKLRTPLNYILLNLAVADLFMVLGGFTSTLYTSLHGYFVFGPTGCNLEGFFATLGGEIALWSLVVLAIERYVVVCKPMSNFRFGENHAIMGVAFTWVMALACAAPPLAGWSRYIPEGLQCSCGIDYYTLKPEVNNESFVIYMFVVHFTIPMIIIFFCYGQLVFTVKEAAAQQQESATTQKAEKEVTRMVIIMVIAFLICWVPYASVAFYIFTHQGSNFGPIFMTIPAFFAKSAAIYNPVIYIMMNKQFRNCMLTTICCGKNPLGDDEASATVSKTETSQVAPA |
Rhodopsin is an important G-protein coupled receptor in phototransduction. Mutation of the rhodopsin gene mainly contributes to various retinopathies such as retinitis pigmentosa. Generally speaking, the protein related to the disease can aggregate with ubiquitin in inclusion bodies, destroy the intermediate filament network, and make up the ability of the cell to degrade non-functioning proteins, which leads to photoreceptor apoptosis. Other mutations in this gene can lead to X-linked congenital stationary night blindness via constitutive activation, especially when the mutations occur around the chromophore binding pocket of rhodopsin. Several other pathological states brought by rhodopsin have also been figured out, including poor post-Golgi trafficking, dysregulated activation, rod outer segment instability, and arrestin binding.
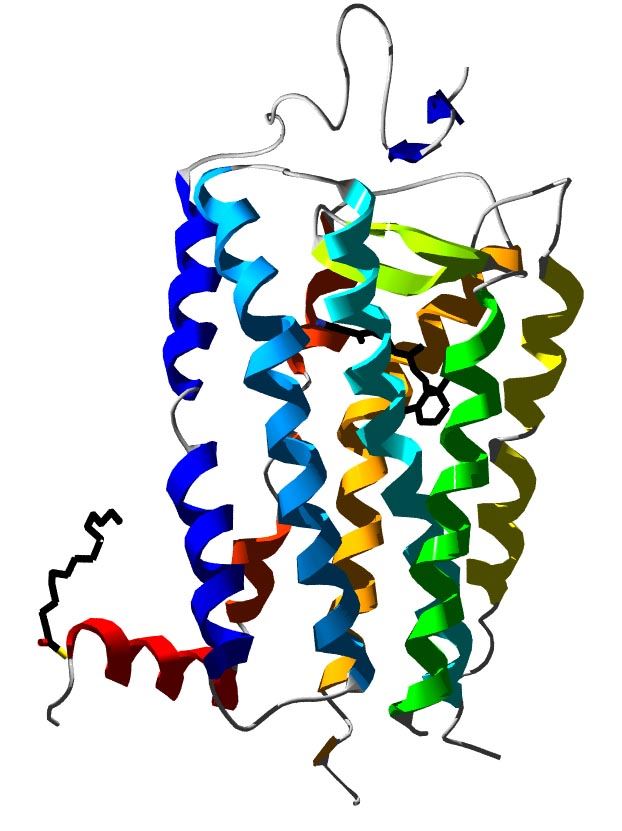 Fig. 1 3D structure of bovine rhodopsin.
Fig. 1 3D structure of bovine rhodopsin.
The article finds that activation of transducin can be prevented by disulfide cross-linking of the helices, which in further suggests the importance of this movement for activation of rhodopsin.
This article demonstrates that archaeal-like rhodopsins are broadly distributed among different taxa. It also indicates that the mode of bacterially mediated energy generation commonly occurs in oceanic surface waters worldwide.
The results suggest that the bond alternation along the unsaturated chain is significant, but weaker in the calculated structure than the one obtained from the X-ray data.
The results show that spectroscopic properties of rhodopsin are similar to those found via bovine rhodopsin in suspension or membrane environment.
This article supports a mechanism that involves pivoting movements of kinked helices, which can be coupled to amplified translation of the helix ends near the membrane surfaces while maintaining hydrophobic contacts in the membrane interior.
We keep in a leading position of membrane protein studies over the past few years. Based on our versatile Magic™ membrane protein production platform, we can provide a series of advanced membrane protein preparation services in reconstitution forms as well as multiple active formats for worldwide customers. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-RHO antibody development services.
During the past years, Creative Biolabs has successfully generated many functional membrane proteins for our global customers. It’s our responsibility to boost the development of our clients’ programs with our experienced service. For more details, please feel free to contact us.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.