It has been reported that any of drugs have the potential to induce tissue damage which can driver an immune response. Hence, it is necessary to assess the tissue damage induced by a drug during an immunogenicity test for a drug candidate. Creative Biolabs has accumulated a lot of experience in a drug’s immunogenicity assay. We are committed to offering the best tissue damage research services to help you obtain the immunological data related to drug candidates.
Tissue damage includes injury to the muscles, bones, tendons, ligaments, cartilage, and skin. It is caused by many unexpected events that may disrupt the homeostasis of the body causing cell damage, such as traumatic events, chemicals, infections, arthritis, and immunological factors. A number of reports have been suggested that a number of drugs have the potential to cause damage. For example, the statin class of drugs, such as Bayol (also known as cerivastatin), may cause damage to a patient’s muscles. Statin-induced tissue damage can also lead to arrhythmia, heart failure, kidney failure, and even death. Besides, an antibiotic prescribed to treat an upper respiratory infection may lead to tendon ruptures and permanent disabilities. Drug-induced damage to non-target tissue can produce an immune response, further resulted in an inflammatory response. Therefore, it is not enough to test the immunogenicity of the drug itself, and the test should also include the effect of the drug on tissue damage or immune system.
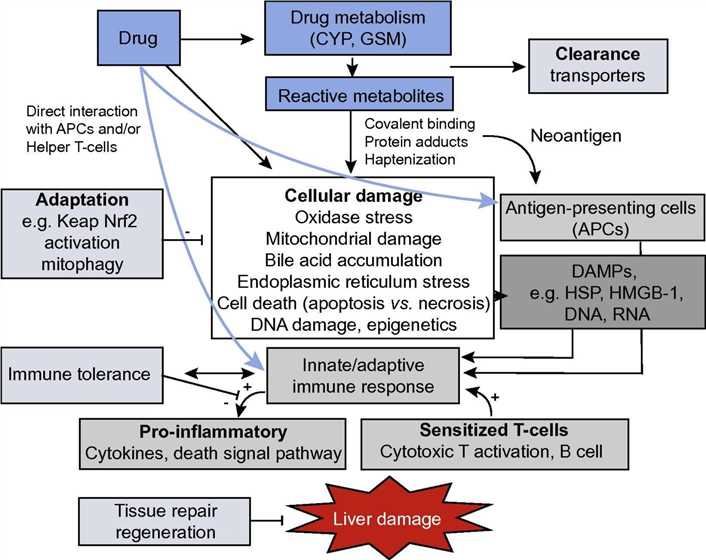 Fig.1 The initiation and progression events relevant to idiosyncratic drug-induced liver injury. (Chen, 2016)
Fig.1 The initiation and progression events relevant to idiosyncratic drug-induced liver injury. (Chen, 2016)
The aim of tissue damage is to determine the damage of a drug to non-target tissue, to predict the possible secondary diseases induced by a drug, and to better understand the potential immunogenicity or cytotoxicity to non-target tissue. The tissue damage study should include:
As a specialist in immunogenicity assay in a preclinical study, Creative Biolabs is confident in providing a wide range of immunogenicity assay services for various drug candidates, including the efficacy and safety of drugs. We have established a comprehensive technology platform that includes a variety of approaches for tissue damage study, such as succinate dehydrogenase activity (MTT assay), microscopy-based histochemistry, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, as well as oxidative stress assay. By MTT assay, we can assess cell metabolic activity. By TUNEL assay, we can detect apoptotic DNA fragmentation and thus identify and quantify apoptotic cells. Our professional scientists also customize an immunogenicity test protocol according to your special purpose.
If you have any problems, please don’t hesitate to contact us for more details.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.