TRPV1, also known as the capsaicin receptor and the vanilloid receptor 1 (VR1), belongs to the transient receptor potential cation channel subfamily V. TRPV1 gene is located at chromosome 17p13.2 and four alternatively spliced transcript variants have been reported. TRPV1 contains 6 transmembrane segments (S1-S6) and exhibits four-fold symmetry around a central ion pathway formed by S5-S6 and the intervening pore loop, which is flanked by S1-S4 voltage-sensor-like domains. TRPV1 is widely expressed in the duodenum, ovary, small intestine, skin, kidney, endometrium, and multiple other tissues.
| Basic Information of TRPV1 | |
| Protein Name | Transient receptor potential cation channel subfamily V member 1 |
| Gene Name | TRPV1 |
| Aliases | G-protein coupled receptor 64, Human epididymis-specific protein 6 |
| Organism | Homo sapiens (Human) |
| UniProt ID | Q8NER1 |
| Transmembrane Times | 6 |
| Length (aa) | 839 |
| Sequence | MKKWSSTDLGAAADPLQKDTCPDPLDGDPNSRPPPAKPQLSTAKSRTRLFGKGDSEEAFPVDCPHEEGELDSCPTITVSPVITIQRPGDGPTGARLLSQDSVAASTEKTLRLYDRRSIFEAVAQNNCQDLESLLLFLQKSKKHLTDNEFKDPETGKTCLLKAMLNLHDGQNTTIPLLLEIARQTDSLKELVNASYTDSYYKGQTALHIAIERRNMALVTLLVENGADVQAAAHGDFFKKTKGRPGFYFGELPLSLAACTNQLGIVKFLLQNSWQTADISARDSVGNTVLHALVEVADNTADNTKFVTSMYNEILMLGAKLHPTLKLEELTNKKGMTPLALAAGTGKIGVLAYILQREIQEPECRHLSRKFTEWAYGPVHSSLYDLSCIDTCEKNSVLEVIAYSSSETPNRHDMLLVEPLNRLLQDKWDRFVKRIFYFNFLVYCLYMIIFTMAAYYRPVDGLPPFKMEKTGDYFRVTGEILSVLGGVYFFFRGIQYFLQRRPSMKTLFVDSYSEMLFFLQSLFMLATVVLYFSHLKEYVASMVFSLALGWTNMLYYTRGFQQMGIYAVMIEKMILRDLCRFMFVYIVFLFGFSTAVVTLIEDGKNDSLPSESTSHRWRGPACRPPDSSYNSLYSTCLELFKFTIGMGDLEFTENYDFKAVFIILLLAYVILTYILLLNMLIALMGETVNKIAQESKNIWKLQRAITILDTEKSFLKCMRKAFRSGKLLQVGYTPDGKDDYRWCFRVDEVNWTTWNTNVGIINEDPGNCEGVKRTLSFSLRSSRVSGRHWKNFALVPLLREASARDRQSAQPEEVYLRQFSGSLKPEDAEVFKSPAASGEK |
As a non-selective ion channel, TRPV1 is involved in a wide range of processes including nociception, thermosensation, and energy homeostasis. The primary role of TRPV1 is to detect and subsequently regulate body temperature. TRPV1 can be activated by a wide number of endogenous and exogenous chemical and physical stimulus, including a temperature above 43°C (109°F), acidic conditions, allyl isothiocyanate, the pungent compound in wasabi and mustard, and capsaicin. The TRPV1 activation results in a painful, burning sensation. The reported endogenous activators include low pH, N-oleyl-dopamine, the endocannabinoid anandamide, and N-arachidonoyl-dopamine. TRPV1 receptors are presented primarily in the nociceptive neurons of the peripheral nervous system and the central nervous system. TRPV1 plays a crucial role in the modulation and transmission of pain sensation, and it is also responsible for the integration of diverse painful stimuli. This receptor is also activated by increases in temperature in the noxious range, suggesting that it functions as a transducer of painful thermal stimuli in vivo.
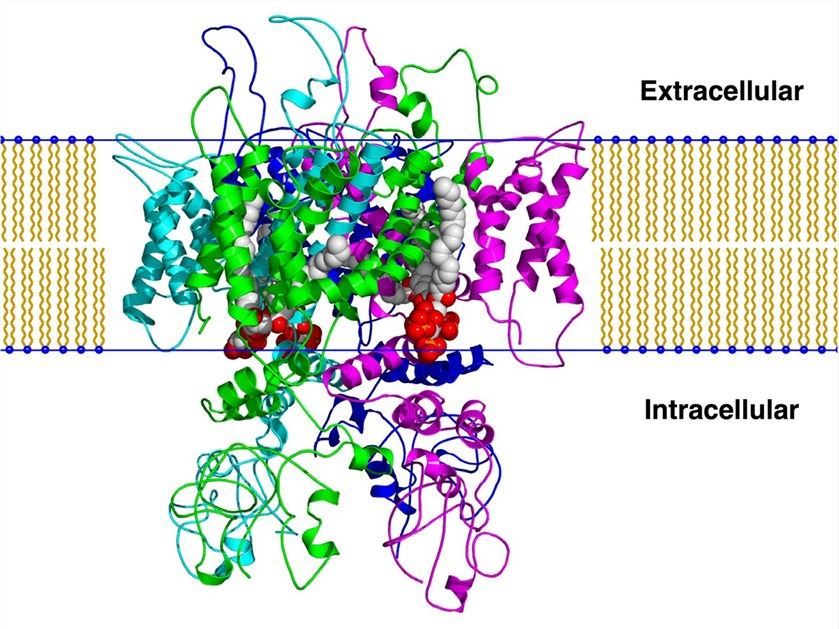 Fig.1 The simulated model of TRPV1 based on a homology model. (Brauchi, 2007)
Fig.1 The simulated model of TRPV1 based on a homology model. (Brauchi, 2007)
This article suggests that TRPV1 ion channels are key components in the development of stress-induced bladder dysfunction, both with regard to bladder wall decompensation that results in the underactive bladder, and increased sensory outflow that results in overactive bladder.
This article reveals that TRPV1 receptor may be associated with the modulation of memory and learning functions, and suggests that activated TRPV1 channels may be potential therapeutic targets for treatment of learning and memory impairments following biliary cirrhosis.
This article confirms that activated TRPV1 ion channel plays anti-oxidative stress and anti-inflammatory role in preventing renal tissue damage and salt-induced hypertension after ischemia-reperfusion injury, and indicates that TRPV1 conveys preconditioning protection that may have a therapeutic implication.
This article confirms the characterization and the identification of TRPV1 receptor in primary canine keratinocytes cultures.
This article concludes that TRPV1 ion channels are involved in neurotoxicity after ischemia and 20-HETE's reactive oxygen species generation.
In order to provide a high-quality membrane protein preparation service, we have developed the versatile Magic™ membrane protein production platform. Our experienced scientists will do their best to help you find a perfect match in your required formats. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-TRPV1 antibody development services.
Creative Biolabs is dedicated to providing first-class membrane protein production service using a variety of strategies. Based on our leading-edge platform, we have successfully produced, purified, stabilized and characterized many challenging membrane protein targets for global customers. If you are interested in the service we can provide, please feel free to contact us for more information.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.