Cytomegalovirus Vaccines
Creative Biolabs is a world leader in the field of viral vaccine development. With our extensive experience and advanced platform, we are therefore confident in offering the best vaccine development services for Cytomegalovirus. We guarantee the finest results for our customers all over the world.
Cytomegalovirus (CMV) is from the family of Herpesviridae. Humans and monkeys serve as natural hosts. Diseases associated with CMV include glandular fever and pneumonia. Most mentions of CMV without further specification refer implicitly to human CMV (HCMV, Human Herpesvirus 5, HHV-5). Human CMV is the most studied of all cytomegaloviruses. HCMV must be acknowledged as one of the most successful human parasites. It has learned to survive in its human host by infecting both vertically and horizontally. The virus can be transmitted by either route during primary infection, reinfection or reactivation. At all times the virus causes minimal disability, allowing infected individuals to remain active and so maintain the maximum opportunity of encountering susceptible contacts. The virus is excreted from multiple sites, so contact of varying degrees of intimacy can lead to transmission.
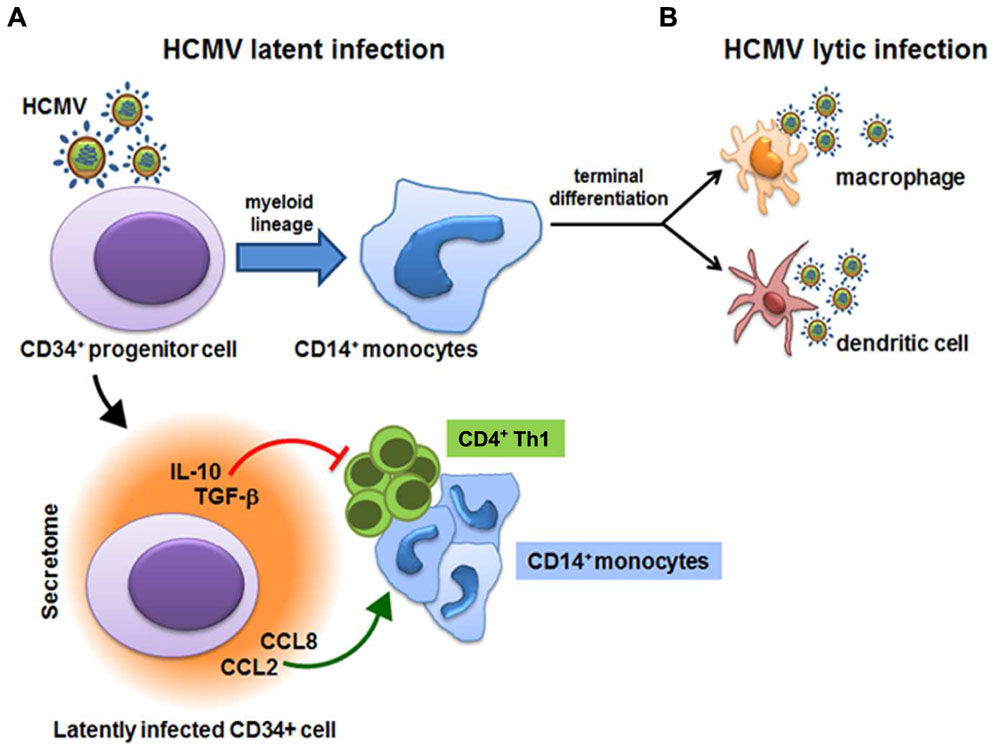 Fig.1 Human Cytomegalovirus (HCMV)-derived secretome and virus latency. 1
Fig.1 Human Cytomegalovirus (HCMV)-derived secretome and virus latency. 1
Pathogenesis for CMV Infection
Primary infection is a major risk factor during pregnancy and for recipients of solid organ transplants but not for bone marrow transplant or AIDS patients. Viraemia has been repeatedly shown to be a risk factor following solid organ or bone marrow transplantation. A high CMV virus load was initially shown to be important in neonates with congenital infection and more recently in renal transplant, liver transplant, bone marrow transplant and AIDS patients. High CMV load is the determinant of CMV disease and that viraemia and donor/recipient serostatus are markers of CMV disease. Studies in liver transplant and bone marrow patients confirm that high CMV load in blood explains the association with donor/recipient serostatus. Furthermore, in all groups of transplant patients, a threshold association with CMV disease is apparent showing that low viral loads may be tolerated by the host.
Host Response to CMV Infection
The responses mounted by the host against different types of CMV infection with normal immunity keep CMV suppressed into a latent state in most individuals for most of the time. Abrogation of these responses permits CMV replication, with full expression of its potential pathogenicity in some cases. Since severe CMV disease is restricted to individuals with impaired cell-mediated immunity, it can be concluded that this arm of the immune response provides most protection against disease. Nevertheless, several lines of evidence suggest that humoral immunity may contribute towards control of CMV, such as CMV disease can be reduced compared to patients receiving placebo or transplacental antibody from the mothers protecting against disease. Severe CMV disease can secondary lead to profound B cell immunodeficiency.
The Development of CMV Vaccine
Cytomegalovirus (CMV) is one of the most significant viral pathogens during pregnancy and in immunocompromised patients. A safe and protective vaccine against CMV is highly desirable in view of the potential positive impact on CMV-associated morbidity and mortality as well as healthcare costs. Unfortunately, this demand could not be met in the past four decades although development of a CMV vaccine has been ranked at the highest priority by the US Institute of Medicine. Multiple different vaccine candidates have been developed and evaluated in phase I clinical trials and few succeeded to phase II trials. Nevertheless, two different vaccines showed recently promising results in trials that studied healthy adults and immunocompromised solid-organ and bone-marrow transplant recipients, respectively. In transplant patients, both two vaccines limited the periods of viraemia and consequently the need for antiviral treatment. The success of these trials is encouraging and will probably give new impetus to the development of an effective CMV vaccine.
Creative Biolabs is pleased to share our cutting-edge technology and extensive expertise in the field of Cytomegalovirus vaccine development and has focused on the viral vaccines for years. We can offer high-quality customized services by adjusting protocols to meet even the most specific requirements. If you are interested in our services, please contact us for more details.
Reference
- Luganini, Anna, Maria E. Terlizzi, and Giorgio Gribaudo. "Bioactive molecules released from cells infected with the human cytomegalovirus." Frontiers in microbiology 7 (2016): 715. Distributed under Open Access license CC BY 4.0, without modification.
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


