Designing mRNA Ebola Vaccines: Delivery Systems and Immunogenicity Analysis
Why mRNA Technology Is a New Hope Against Ebola in the Post-First-Generation Vaccine Era
Filoviruses, including Ebola virus (EBOV), Sudan virus, and Marburg virus, remain a persistent threat to global public health. These pathogens cause severe hemorrhagic fever with high mortality rates, and their potential for rapid spread—exacerbated by limited healthcare infrastructure in endemic regions—highlights the urgent need for flexible and fast-acting vaccine platforms.
The first-generation recombinant vaccine has marked a significant milestone in Ebola prevention. Approved by regulatory authorities globally, this vaccine based on a vesicular stomatitis virus (VSV) vector played a critical role in controlling the 2014-2016 West Africa Ebola outbreak and subsequent epidemics, demonstrating its value as a core public health tool. However, the COVID-19 pandemic showcased the revolutionary potential of mRNA vaccine technology—its speed, adaptability, and potent immunogenicity have redefined how we respond to infectious diseases. This has positioned mRNA as an ideal candidate for developing the next generation of Ebola vaccines.
This article systematically reviews the design strategies, core technologies, and preclinical evidence of mRNA Ebola vaccines, while exploring their future development paths and clinical translation prospects.
The Current Ebola Vaccine Landscape: Achievements and Limitations of Recombinant VSV-Based Vaccines
Mechanism of Action and Clinical Evidence
The recombinant VSV-based Ebola vaccine operates by using a live, attenuated VSV vector engineered to express the Ebola virus glycoprotein (EBOV GP). When administered, the vector infects host cells, triggering the production of EBOV GP, which then stimulates a robust immune response. Clinical trials have confirmed its impressive protective efficacy, ranging from 97.5% to 100% in outbreak settings. Additionally, studies indicate that the antibody responses induced by this vaccine can persist for several years, although neutralizing antibody titers may gradually decline over time.
Platform Limitations: Paving the Way for Next-Generation Vaccines
Despite its success, the recombinant VSV-based platform has notable drawbacks. In terms of safety and reactogenicity, common adverse events include fever, headache, arthritis, and skin rashes. While generally safe for healthy individuals, its use as a live viral vector raises concerns for immunocompromised populations, requiring careful risk assessment.
Production and supply chain challenges further limit its global accessibility. Manufacturing live recombinant viral vaccines involves complex bioprocessing, long production cycles, and strict cold chain requirements—barriers that hinder rapid deployment in resource-limited regions where Ebola outbreaks often occur. These limitations underscore the need for a new vaccine platform with improved safety, simpler production, and greater flexibility.
The mRNA Vaccine Platform: A Technological Cornerstone for Addressing Ebola Prevention Challenges
Core Mechanism of mRNA Ebola Vaccines
A common question arises: "Is the currently approved Ebola vaccine an mRNA vaccine?" The answer is no—existing approved vaccines rely on viral vector technology. However, mRNA technology represents a highly promising direction for next-generation Ebola vaccine development.
The working principle of mRNA vaccines is elegant and precise. mRNA molecules carry genetic information encoding EBOV GP. Delivered via a specialized system, these mRNA molecules enter human cells and utilize the cell's own ribosomes to translate the genetic code into GP proteins. These proteins are then presented to the immune system, activating specific B-cell and T-cell responses. Crucially, this process does not involve viral replication or genomic integration, eliminating associated risks.
Platform Advantages: Why mRNA Technology Excels for Emerging Infectious Diseases Like Ebola
- Rapid Development: mRNA vaccines can be designed and produced in weeks once the viral genome sequence is available—a critical advantage for responding to sudden outbreaks, where every day counts.
- Scalable Production: The in vitro transcription (IVT) process for mRNA manufacturing is a standardized, cell-free system, enabling large-scale production quickly and efficiently without the need for complex cell culture facilities.
- Enhanced Safety: As non-viral, non-replicating, and non-integrating agents, mRNA vaccines avoid the potential risks associated with live viral vectors, such as reversion to virulence or insertional mutagenesis.
- Flexibility and Multivalent Potential: mRNA sequences can be easily modified to target viral variants. Additionally, multiple mRNA molecules encoding GP proteins from different filoviruses (e.g., Ebola, Sudan, Marburg) can be combined to create "pan-filovirus" vaccines, offering broad protection against related pathogens.
Design and Optimization: Key Steps in Building Effective mRNA Ebola Vaccines
Antigen Design: Selecting and Optimizing Ebola Virus Glycoprotein
EBOV GP is widely recognized as the "golden target" for vaccine design. As the key protein mediating viral entry into host cells, it is the primary target of neutralizing antibodies, making it indispensable for inducing protective immunity.
To maximize vaccine efficacy, extensive mRNA sequence engineering is required:
- Codon Optimization: Adjusting the mRNA sequence to match the codon usage preferences of human cells significantly enhances the expression level of GP proteins.
- UTR and Poly(A) Tail Design: The 5' and 3' untranslated regions (UTRs) and the Poly(A) tail play crucial roles in improving mRNA stability and translation efficiency, ensuring the sustained production of antigenic proteins.
- Nucleoside Modification: Incorporating modified nucleosides such as pseudouridine reduces the inherent immunogenicity of mRNA (avoiding excessive activation of TLR7/8 pathways) while boosting translation efficiency, minimizing unwanted inflammatory responses and enhancing vaccine performance.
Delivery Systems: In-Depth Analysis of Lipid Nanoparticle (LNP) Technology
LNPs are not merely protective "shells" for fragile mRNA molecules—they are active delivery systems that determine vaccine success. Their unique structure enables efficient delivery of mRNA to target cells, overcoming biological barriers.
The core components of LNPs and their functions include:
- Ionizable Lipids: The "engine" of LNP technology. These lipids protonate in the low-pH environment of endosomes, disrupting the endosomal membrane and facilitating the release of mRNA into the cytoplasm.
- Helper Phospholipids (e.g., DSPC): Acting as structural scaffolds, these lipids maintain the stability and morphology of LNPs, ensuring consistent performance.
- Cholesterol: Regulates membrane fluidity, fills lipid gaps, and enhances LNP stability, prolonging their circulation time in the body.
- PEGylated Lipids: Form a hydrophilic layer on the LNP surface, reducing clearance by the reticuloendothelial system, extending systemic circulation, and controlling particle size.
For Ebola mRNA vaccines, LNP formulation optimization focuses on three key areas: improving delivery efficiency to antigen-presenting cells (e.g., dendritic cells), enhancing adjuvant effects to boost immune responses, and improving thermal stability to suit deployment in resource-limited regions like Africa. Preclinical studies have confirmed that mRNA-EBOVgp vaccines delivered via LNPs exhibit strong efficacy.
Services you may interested in
Immunogenicity and Protective Efficacy: Strong Evidence from Preclinical Studies
Inducing Potent and Durable Humoral Immunity
Preclinical studies in animal models (primarily mice and non-human primates) have consistently shown that single or two-dose immunization with LNP-mRNA-EBOVgp vaccines induces high titers of functional neutralizing antibodies—exceeding the levels observed in the serum of convalescent patients. These antibodies specifically target EBOV GP, blocking viral entry into host cells. Additionally, existing data indicate that vaccine-induced antibody responses are durable, providing a foundation for long-term protection.
Activating Broad-Spectrum T-Cell Immune Responses
mRNA Ebola vaccines effectively induce Th1-biased CD4+ T-cell responses, which are critical for supporting B cells to produce high-quality antibodies and regulating overall immune responses. Furthermore, these vaccines stimulate robust cytotoxic CD8+ T-cell (CTL) activity. CTLs recognize and eliminate virus-infected cells, forming a second line of defense against Ebola infection—complementing humoral immunity and enhancing overall protection.
Complete Protection in Animal Challenge Models
Key preclinical studies have demonstrated the protective efficacy of mRNA Ebola vaccines. Animals immunized with mRNA-EBOVgp showed 100% survival following challenge with a lethal dose of Ebola virus, while all unvaccinated control animals succumbed to infection. This direct evidence confirms the vaccine's ability to prevent severe disease and death, validating its potential for clinical application.
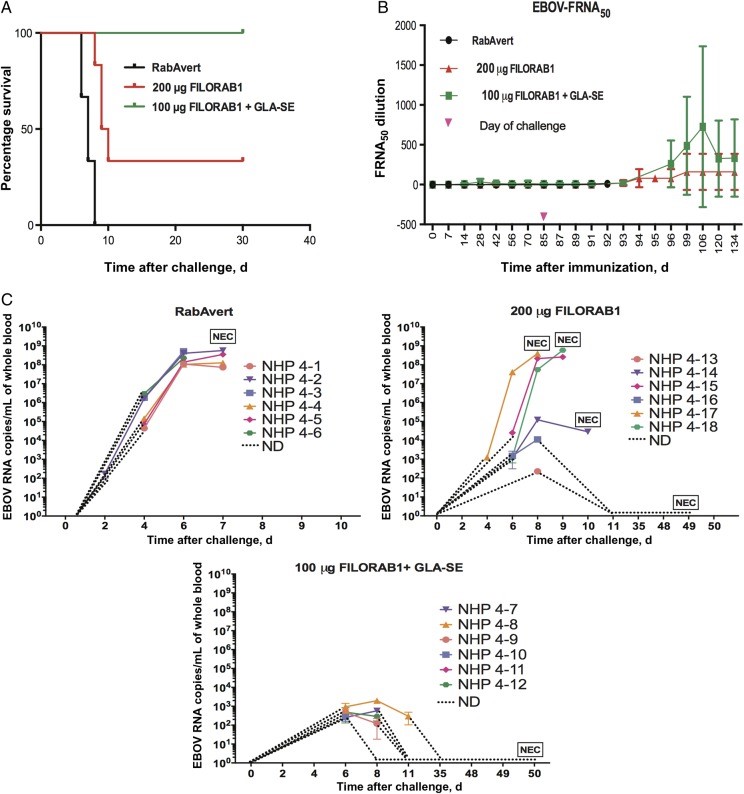 Fig.1 Survival, Neutralizing Antibody Titers, and Viral RNA Loads in Immunized NHPs Following Ebola Virus Challenge.1,2
Fig.1 Survival, Neutralizing Antibody Titers, and Viral RNA Loads in Immunized NHPs Following Ebola Virus Challenge.1,2
Comprehensive Comparison: Recombinant VSV-Based Vaccines vs. mRNA Ebola Vaccine Candidates
| Characteristics | Recombinant VSV-Based Vaccines | mRNA Ebola Vaccine Candidates |
| Technology Platform | Live recombinant viral vector | Non-viral, synthetic mRNA/LNP platform |
| Mechanism of Action | Live virus infects cells to express antigen | mRNA translates antigen in the cytoplasm |
| Immunogenicity | Potent neutralizing antibody responses; rapid onset | Strong humoral and cellular immune responses |
| Safety | Moderate reactogenicity; caution in immunocompromised populations | Local reactions common; systemic reactions mild and transient |
| Manufacturing | Cell culture-dependent; complex process; long cycle | Cell-free in vitro transcription; rapid; highly scalable |
| Deployment Flexibility | Strict cold chain requirements; monovalent | High cold chain demand (potential for improvement); easy to develop multivalent vaccines |
| Regulatory Status | Approved by global regulatory authorities | Preclinical/early clinical development stages |
Differences in Mechanism and Immune Profiles
Recombinant VSV-based vaccines simulate natural infection by using a live vector to infect host cells, activating innate immune pathways through viral replication signals. In contrast, mRNA vaccines deliver precise genetic information without replication, focusing on targeted antigen expression and controlled immune activation. This difference results in distinct immune profiles: viral vector vaccines excel at inducing rapid antibody responses, while mRNA vaccines elicit balanced humoral and cellular immunity, potentially offering broader and more durable protection.
Trade-Offs in Safety and Reactogenicity
Recombinant VSV-based vaccines have a well-established safety profile in tens of thousands of individuals, but their reactogenicity—including occasional severe arthralgia—cannot be ignored. mRNA vaccines, validated in hundreds of millions of people through COVID-19 vaccination, have a different adverse event profile: most reactions are mild (e.g., injection site pain, low-grade fever) and resolve quickly. The non-replicating nature of mRNA vaccines also eliminates risks associated with live vectors, making them safer for vulnerable populations.
Production, Cost, and Global Accessibility
mRNA technology's production advantages translate to improved global accessibility. The cell-free IVT process reduces manufacturing complexity and costs, enabling rapid scaling to meet global demand. This is particularly critical for resource-limited regions, where simple production and reduced cold chain dependence (with future LNP improvements) could enhance vaccine availability during outbreaks. In contrast, the complex biomanufacturing of viral vector vaccines limits their ability to respond quickly to sudden epidemics.
Conclusion and Outlook: How mRNA Technology Will Reshape Global Filovirus Prevention
mRNA technology offers unparalleled advantages in speed, flexibility and safety for next-gen Ebola vaccines, with solid preclinical data supporting its clinical transition. It will complement rather than replace existing vaccines. Future focus includes clinical trial results, LNP thermal stability improvements, and pan-filovirus vaccine development. Ultimately, it will reshape global prevention and response strategies against filoviruses and other emerging infectious diseases.
If you want to learn more about the norovirus vaccine, please refer to:
Ebola Virus Glycoprotein Structure-Entry Mechanism-and Therapeutic Implications
Ebola Virus Replication Cycle Host Factors-RdRp Kinetics-and Vaccine Targets
Browse our Norovirus Antigen Products
→ Ebola Virus - Viral Antigens
Need a custom solution? If our off-the-shelf products aren't a perfect fit, we can create one for you. Contact us to design a product that precisely matches your experimental demands.
References
- Johnson, Reed F., et al. "An inactivated Rabies virus–based Ebola Vaccine, FILORAB1, adjuvanted with glucopyranosyl lipid A in stable emulsion confers complete protection in nonhuman primate challenge models." The Journal of infectious diseases 214.suppl_3 (2016): S342-S354. https://doi.org/10.1093/infdis/jiw231
- Distributed under Open Access license CC BY 4.0, without modification.
Created December 2025
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


