The Translational Value of BRSV Models in Human RSV Vaccine
The Global Health Challenge of RSV and the Quest for the Ideal Model
Respiratory Syncytial Virus (RSV) remains a leading global cause of severe pneumonia, particularly in infants and the elderly. The urgent need to find reliable animal models is paramount to accelerate the research and development (R&D) of human RSV (HRSV) vaccines. Among the preclinical options, the Bovine RSV (BRSV) model stands out as a highly relevant natural model. It exhibits significant similarities to HRSV in structure, antigenicity, and pathogenesis. Because of these shared characteristics, including gene sequence, the conformation of the fusion (F) and attachment (G) proteins, and the disease progression, BRSV is considered an "excellent model" for assessing HRSV vaccine candidates.
Overview of the BRSV Model
The BRSV model utilizes both naturally infected cattle and experimentally infected calves. Belonging to the Paramyxoviridae family, like HRSV, the virus's F protein maintains a highly conserved pre-fusion conformation.
Key Advantages and Disadvantages
| Category | Key Takeaways |
|---|---|
| Model Animals | Naturally infected adult cattle and experimentally infected calves. |
| Experimental Advantages | The disease course and clinical manifestations, such as bronchiolitis and pneumonia, closely mirror the human condition, allowing for large-scale immunological and pathological assessments. |
| Preclinical Gold Standard | BRSV infection in calves replicates the bronchiolitis and pneumonia pathology seen in human RSV cases, establishing it as a "preclinical gold standard" for evaluating vaccine immunogenicity and safety. |
| Limitations | Higher costs, significant facility requirements, and the need for cross-species correction for certain immunological differences. |
Services you may interested in
The High Degree of Similarity Between BRSV and HRSV
The translational value of the BRSV model is rooted in the remarkable homology between the bovine and human viruses.
- Genetic and Protein Homology: The pre-fusion structure of the F protein shares over 90% homology, a critical factor since this structure is the primary target for neutralizing antibodies. The overall gene similarity is approximately 80%.
- Pathogenic Mechanism: The disease process is consistent: the virus initially infects epithelial cells in the upper respiratory tract before spreading to the lower respiratory tract, leading to airway obstruction and inflammation. Experimental observations in BRSV align with the detailed replication pathways and immune evasion mechanisms described for RSV in the literature.
- Immune Response: The immune response generated in calves infected with BRSV also shows a high degree of overlap with that in humans infected with HRSV. Calves produce neutralizing antibodies and cellular immunity that are comparable to those in humans. This similarity in immune response allows researchers to use the BRSV model to study the immune effects of potential vaccines, providing valuable information on how the human immune system might respond to an HRSV vaccine.
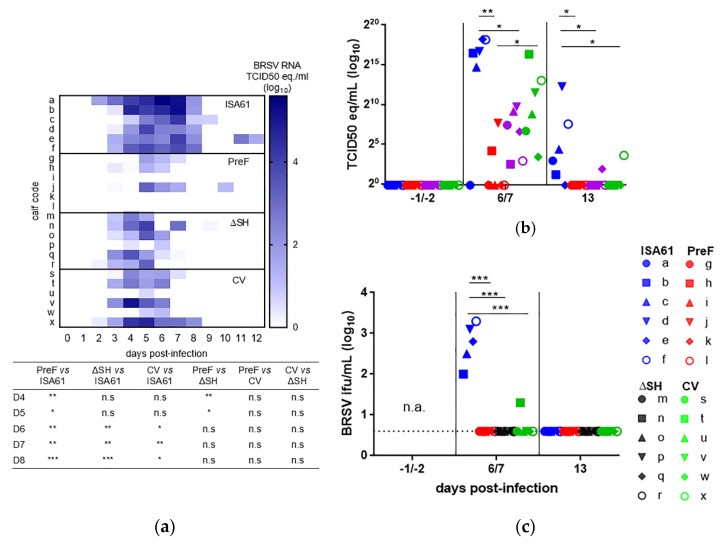 Fig.1 Vaccination Significantly Reduces BRSV Viral Load In the Upper and Lower Respiratory Tracts of Challenged Calves.1,2
Fig.1 Vaccination Significantly Reduces BRSV Viral Load In the Upper and Lower Respiratory Tracts of Challenged Calves.1,2
Practical Applications of BRSV in Human RSV Vaccine Development
The BRSV model has been widely applied in various aspects of human RSV vaccine development, providing critical data to support the advancement of vaccine candidates.
In a 2023 European interdisciplinary collaboration study, researchers aimed to evaluate the immunogenicity of a new mRNA RSV vaccine using the BRSV calf model. The key finding was that calves vaccinated with the mRNA vaccine produced high titers of prefusion F neutralizing antibodies. This result suggests that similar mRNA vaccines may have the potential to induce the production of protective antibodies in pregnant women, which can then be transferred to the fetus through the placenta, providing passive immunity to newborns.
Another important application is seen in the early animal experiments of an mRNA vaccine. The goal of these experiments was to verify the safety of the mRNA vaccine and its dose-response relationship. The BRSV calf model showed no evidence of enhanced disease, a major concern in RSV vaccine development. This positive outcome supported the progression of the vaccine to Phase I/II clinical trials, bringing it one step closer to potential use in humans.
Additionally, a study comparing live virus and subunit vaccines for their protective efficacy utilized the BRSV model. After inoculation with BRSV-based vaccine candidates, significant reductions in viral load and lung tissue pathology scores were observed. These results provided a basis for selecting the most effective vaccine formulation, helping researchers make informed decisions about which type of vaccine to further develop.
Collectively, these cases demonstrate that the BRSV model can provide translatable data in three key areas: immunogenicity assessment, dose optimization, and evaluation of the risk of enhanced disease. This data is essential for guiding the development of HRSV vaccines and increasing the likelihood of successful clinical outcomes.
A Review of the History of RSV Vaccine Development
The journey of RSV vaccine development has been complex:
- Early Attempts (1960–1990): Efforts with inactivated and live-attenuated vaccines unfortunately led to enhanced pneumonia in some recipients, shifting the research focus toward safer subunit/protein vaccines.
- The Structural Biology Breakthrough (2013–2017): Advances in structural biology revealed that the unstable pre-fusion conformation of the F protein is the target for highly potent neutralizing antibodies. This discovery was a pivotal moment, driving modern vaccine design.
The commercialization of vaccines has been swift since this breakthrough:
- First Approvals (2023–2024): Modern vaccines based on the pre-fusion F protein have reached the market. These include pre-fusion F bivalent vaccines approved for maternal immunization to protect infants, with one showing protection against severe RSV in infants of approximately 70%, and the first mRNA RSV vaccine approved for older adults ≥ 60 years of age.
- Passive Immunization: A long-acting monoclonal antibody targeting the F protein is also available for passive immunization in newborns and high-risk infants, offering immediate and prolonged protection with a single dose covering the entire RSV season.
Overview of the Current RSV Vaccine Pipeline (as of 2025)
The global RSV vaccine market is projected to exceed $2 billion by 2024, primarily fueled by maternal vaccines and long-acting monoclonal antibodies. The current pipeline demonstrates diverse strategies:
| Type/Formulation | Development Stage | Target Population | Key Characteristics |
|---|---|---|---|
| Bivalent pre-fusion F Subunit Vaccine | Marketed (2023) | Pregnant Women → Neonates | Induces high titers of pre-fusion F neutralizing antibodies via an advanced adjuvant system. |
| Monovalent pre-fusion F Subunit Vaccine | Marketed (2024) | Pregnant Women → Neonates | Utilizes a distinct emulsion adjuvant to enhance antibody affinity and duration. |
| mRNA Vaccine | Marketed (2024) | ≥ 60 Year Old Adults | Based on a Lipid Nanoparticle (LNP) delivery platform, it rapidly expresses the pre-fusion F antigen in vivo, significantly reducing RSV cases in the elderly. |
| Long-Acting Monoclonal Antibody | Marketed (2023) | Neonates/High-Risk Infants | Targets a highly conserved epitope on the F protein, providing immediate and season-long protection. |
| Recombinant Protein/Nanoparticle Vaccine | Phase III Clinical Trial | Pregnant Women | Uses self-assembling nanoparticle technology to boost antigen presentation and mucosal immunity, aiming for broader coverage against different RSV subtypes. |
| Oral/Nasal Mucosal Vaccine | Phase I/II Clinical Trial | Children, Elderly | Explores mucosal immunity for easier administration and to induce local IgA immunity. |
Assessing the Translational Value of BRSV
The BRSV model serves as a critical translational bridge in the R&D process.
| Assessment Dimension | Specific Performance in BRSV Model | Value Proposition |
|---|---|---|
| Immunogenicity Prediction | Neutralizing antibody titers produced in BRSV calves are comparable to human sera. | Provides quantitative data for setting optimal clinical doses. |
| Safety Screening | Absence of enhanced pneumonia (ADE) and low pathological scores. | Reduces early safety risks in clinical trials. |
| Vaccine Platform Comparison | Simultaneous evaluation of live virus, subunit, and mRNA platforms is possible. | Offers a horizontal comparison for selecting the optimal R&D pathway. |
| Cost-Effectiveness | While facility costs are high, the large number of animals per experiment and strong data reproducibility. | Long-term reduction of R&D failure rates and improved project returns. |
Future Directions and Challenges
To further enhance the model's utility, the R&D community is focusing on several areas:
- Standardizing Cross-Species Immunology: Establishing conversion models between calf serum neutralizing antibody titers and human serum titers.
- Genetic Engineering: Utilizing tools to generate BRSV strains that better mimic human pathogenesis, such as replicating specific circulating mutant strains.
- Multipathogen Evaluation: Simultaneously comparing the protective efficacy of different platforms (mRNA, protein subunits, monoclonal antibodies) within the same BRSV experiment.
- Data Sharing: Promoting the sharing of BRSV experimental data across international research institutions to improve model reproducibility.
Conclusion
The BRSV model, with its profound structural and immunological similarity to HRSV, is firmly established as a key translational bridge in human RSV vaccine development. It allows R&D teams to obtain reliable preclinical data in the core areas of immunogenicity, dose optimization, and enhanced disease risk assessment, significantly improving the odds of clinical success. As pre-fusion F vaccines and mRNA platforms continue their rapid commercialization, the value of BRSV will only increase in the screening of new immunomodulators, the comparison of vaccine platforms, and the development of next-generation vaccines.
If you want to learn more about the norovirus vaccine, please refer to:
Understanding the Molecular Mechanisms of Three RSV Vaccine Construction Strategies
Respiratory Syncytial Virus F Protein in Next-Generation Vaccine Design
RSV Infection Life Cycle and Vaccine Target Discovery
Deciphering the Molecular Mechanisms of Respiratory Syncytial Virus Infection
Browse our Norovirus Antigen Products
Need a custom solution? If our off-the-shelf products aren't a perfect fit, we can create one for you. Contact us to design a product that precisely matches your experimental demands.
References
- Valarcher, Jean François, et al. "Single-shot vaccines against bovine respiratory syncytial virus (BRSV): comparative evaluation of long-term protection after immunization in the presence of BRSV-specific maternal antibodies." Vaccines 9.3 (2021): 236. https://doi.org/10.3390/vaccines9030236
- Distributed under Open Access license CC BY 4.0, without modification.
Created October 2025
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


