Vaccines for Virus from Adenoviridae Family
Creative Biolabs provides global solutions for worldwide customers in the field of viral vaccine development. We have successfully accomplished many projects in viral vaccine development services for the virus from adenoviridae family. We guarantee the finest results for our customers all over the world.
Adenovirus is a type of uncoated particles with a diameter of 70~90 nm. They are linear double-stranded DNA molecules, about 4.7 kb, with reverse repeats of about 100 bp at each end. Since the 5' ends of each DNA strand are covalently bound to a protein molecule with a relative molecular weight of 55 plus 10Da, the circular structure of double-stranded DNA can be formed. More than 100 serotypes have been found successively, among which 52 human adenoviruses are divided into six subgroups (A, B, C, D, E and F). Adenovirus can infect the respiratory tract, gastrointestinal tract, urethra, bladder, eye, and liver. About a quarter of known human adenovirus serotypes are usually associated with human diseases, and each serotype can cause different clinical diseases. In contrast, different serotypes can cause the same disease.
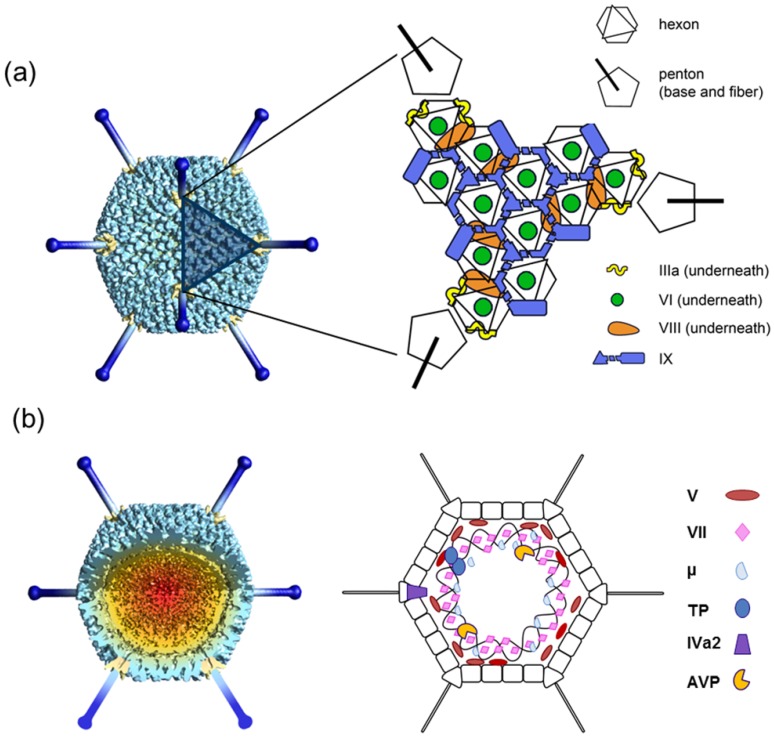 Fig. 1 Overall AdV structure and components. 1
Fig. 1 Overall AdV structure and components. 1
Canine Adenovirus Type 1 Vaccine
Canine adenovirus (CAV) is the most pathogenic virus in mammalian adenovirus genus. CAV-1 can cause canine infectious hepatitis, (necrosis at the center of the hepatic lobule, liver parenchyma cell and cortex of inclusions and bleeding time in the nuclei of acute septic), and can also cause fox encephalitis. The disease is not only prevalent in domestic dogs and foxes in China and around the world, but also widely popular in wild foxes, bears, coyotes and raccoons. The disease can occur all year round.
Canine Adenovirus Type 2 Vaccine
Canine adenovirus can infect and replicate in epithelial cells of respiratory tracts, gastrointestinal tracts, urinary bladder and eyes. CAV-2 primarily infects children and accounts less in adults. CAV-2 is mainly transmitted through direct (nasal to nasal) and indirect contact with the nasal secretions of infected dogs. The studies indicate that safety management and vaccination against CAV-2 are key parts of the control and prevention of this disease.
With the advantages of rapid preparation, low cost, safety and easy storage and transport, the vaccine has played a great role in the treatment of Canine adenovirus type 2. The vaccine which is made from infected dog liver tissue has significant effects on preventing infectious hepatitis syndrome.
Adenovirus Type 4 and Type 7 Vaccine
Adenovirus type 4 and type 7 vaccines are widely used in various adenovirus-related disease prevention. Adenovirus produces three types of infections, Lytic infection, Latent infection and Oncogenic transformation. Adenovirus infection produces large amounts of adenovirus proteins that contribute to the high expression of heterologous genes. The translation of potentially exogenous proteins is found in the adenovirus system. The adenovirus type 4 and type 7 vaccine have been approved to be safe and effective in many cases.
Adenovirus Type 5 Vaccine
Adenovirus type 5 causes primarily infections of the upper respiratory tract infection and pneumonia. It is mainly characterized by stuffiness (nasal congestion), runny nose, low-grade fever, post-nasal drip, and cough. The disease is highly contagious and mortality rate, which can spread throughout the year. The vaccine which is made from infected dog liver tissue has significant effects on preventing Adenovirus type 5 related diseases.
Our Services for Viral Vaccines from Adenoviridae Family
Our platform provides a seamless, high-quality, single-source value chain from discovery to commercialization in adenoviridae family project. As a research-driven and customer-focus company, we are dedicated to helping our worldwide customers shorten the discovery and development time and lower the cost of viral vaccine development process.
- Mature vaccine development system
- Experienced design and solutions to viral vaccine development
- Efficient and time-saving discovery system
Creative Biolabs is a professional vaccine development expert with extensive experience in the viral vaccine development services for years. We have experienced experts and advanced platforms that can provide excellent services. If you are interested in our services, please contact us for more details.
Reference
- San Martín, Carmen. "Latest insights on adenovirus structure and assembly." Viruses 4.5 (2012): 847-877. Distributed under Open Access license CC BY 3.0, without modification.
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


