As a pioneer in the antibody development field, Creative Biolabs focuses on offering high-quality and innovative in vitro diagnostic (IVD) antibody & immunoassay development services to further serve the diagnosis market. Our service portfolio targets a wide spectrum of biomarkers of all types of cancer, such as testicular cancer.
Testicular cancer is cancer that develops in the testicles. It can start in one or both testicles and occurs most frequently in young men. The most common manifestation of testicular cancer is a painless, hard swelling, or enlargement of the testicle. Although the etiology of testicular cancer is unknown, several risk factors such as undescended testis, family history of the disease, and previous history of testicular cancer could contribute to its development. Testicular cancer is highly curable and usually curable. Generally, orchiectomy is the first step in treating testicular cancer and the tissues are also helpful in determining the pathologic diagnosis. Besides, chemotherapy including bleomycin, etoposide, and cisplatin (BEP) has been used to treat testicular cancer since the 1970s. Other treatments including radiation therapy and adjuvant therapy are used for different stages or purposes.
Testicular tumors can be broadly classified into four groups: germ cell tumors, sex cord-stromal tumors, mixed germ cell/sex cord-stromal tumors, and miscellaneous tumors. Different types of testicular tumors are treated differently, therefore an accurate diagnosis is of crucial importance for patient management. Generally, in any patient with a testicular mass, or unexplained scrotal pain or swelling, an ultrasonogram of the scrotum should be obtained first, which helps distinguish between intratesticular and extratesticular pathology. Thereafter, a computed tomographic (CT) scan is used to locate metastases. For the differential diagnosis of testicular cancer, the histological examination of the tissue obtained from the orchiectomy is required. However, biopsy through the scrotum is not recommended because it may increase the incidence of spread to the scrotum or regional lymph nodes.
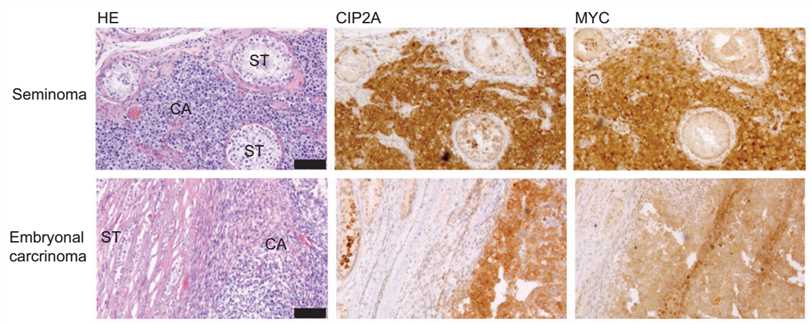 Fig.1 CIP2A, MYC immunohistochemical staining in two different human testicular cancer samples, seminoma, and embryonal carcinoma.1
Fig.1 CIP2A, MYC immunohistochemical staining in two different human testicular cancer samples, seminoma, and embryonal carcinoma.1
In most cases, an accurate diagnosis of testicular cancer can be achieved based on careful examination of the morphology of the lesion and its surrounding tissues. However, in some cases, the differential diagnoses might present as a difficult challenge for pathologists due to their similarities such as in tumor architecture. For these particularly problematic cases, immunohistochemical staining has proved of significant value. Traditional markers such as placental-like alkaline phosphatase and alpha-fetoprotein (AFP) still play a role. More importantly, many new markers that have greater sensitivity and specificity for the differential diagnosis of testicular cancer have been discovered and proved to be key ancillary tools.
Creative Biolabs have established a thorough custom IVD antibody & immunoassay development procedure that takes into account every aspect of your research to deliver adaptive assay solutions to suit your specific requirements. The procedure includes assay design, protein production, antibody development, antibody conjugation, antibody pairing, assay establishment, assay validation, etc. For more detailed information, please click the following links:
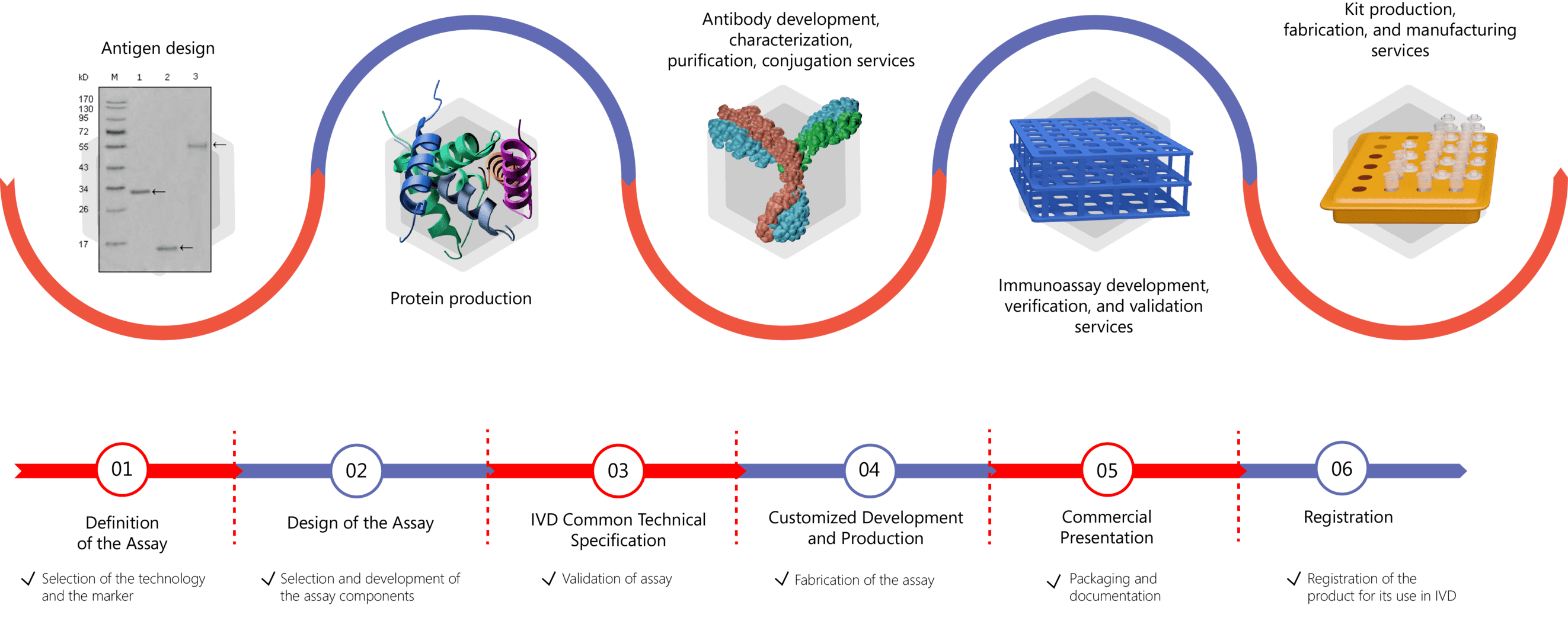
Our services can be tailored targeting different biomarkers with potential for testicular cancer diagnosis and prognosis, including but not limited to:
Please feel free to contact us for more information and discuss your project needs.
Reference
For Research Use Only.
