With decades of experience in antibody generation, Creative Biolabs offers customized antibody development services for research, diagnostic, and therapeutic applications. Here, we introduce our in IVD (in vitro diagnostic) antibody development services to promote and accelerate your IVD project development. Especially, we offer CA 11-19-specific antibody development services for colorectal cancer screening and treatment monitoring.
Tumor antigen (CA 11-19) is a tumor antigen, which is identified as a protein expressed on the surface of certain cancer cells. Recent amino acid sequencing data indicate that the 100 kDa, CA 11-19 antigen is a 701 amino acid glycoprotein. CA 11-19 is not the cause of cancer; conversely, it is shed by the cancer cells and can be detected by laboratory tests in blood and sometimes other body fluids. CA 11-19 is increased in approximately 70% to 95% of patients with advanced pancreatic cancer. Moreover, CA 11-19 may also be increased in other cancer types and diseases including: colorectal cancer, cholangiocarcinoma (gallbladder and bile duct cancers), liver cancer, gastric cancers, lung cancer, ovarian cancer, bile duct obstruction (e.g., gallstones), pancreatitis, cystic fibrosis, liver disease, and thyroid disease.
Colorectal cancer (CRC) remains the second most common cause of cancer deaths in both sexes in the United States and Europe. Early detection of colon cancer is crucial to improving survival rates, because over 90% of those diagnosed with early-stage CRC patients live 5 or more years after treatment, and screening procedures play an essential role for earlier diagnosis. A noninvasive, convenient, accurate test with higher sensitivity and specificity is needed for CRC to improve screening rates. Data was recently reported on the new serum blood test colorectal tumor marker CA 11-19. CA 11-19 was shown to be a serologic tumor marker for colorectal cancer with a sensitivity and specificity of 97.7% and 84.4% respectively. It has been reported that CA 11-19 does not cross-react with carcinoembryonic antigen (CEA) antibodies.
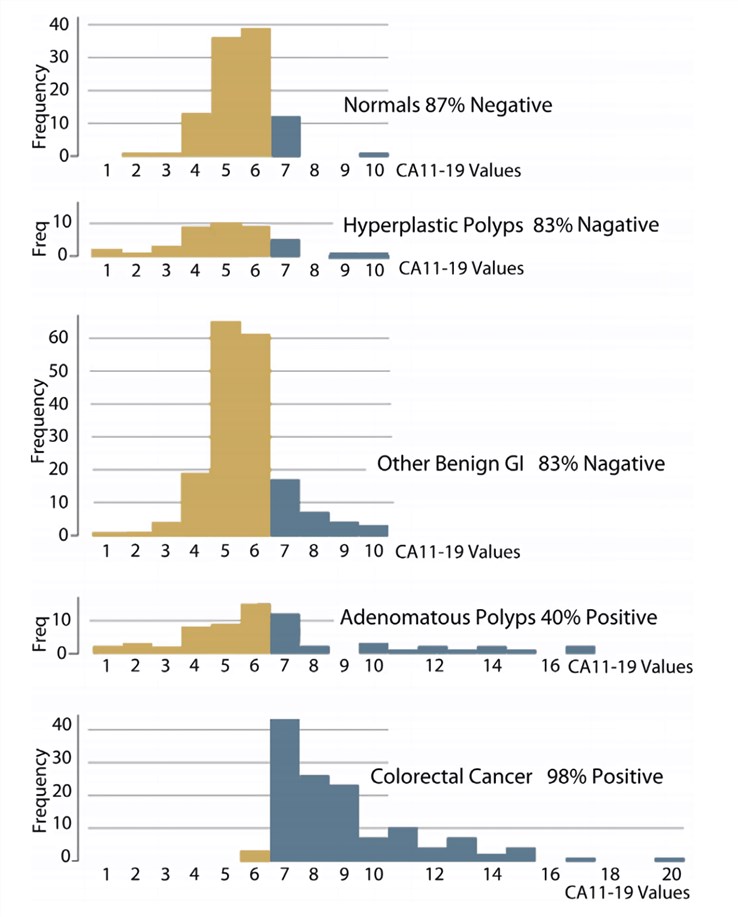 Fig.1 Histograms of the CA 11-19 assay values for the 522 colonoscopy-confirmed subjects. (Overholt, B. F., 2016)
Fig.1 Histograms of the CA 11-19 assay values for the 522 colonoscopy-confirmed subjects. (Overholt, B. F., 2016)
Nowadays, IVD antibodies against CA 11-19 can be developed to help the prognosis and diagnosis of colorectal cancer. As a world-leading company in the field of IVD antibody development, Creative Biolabs has launched a whole series of biomarker-specific antibody development services, with the ability to serve clients around the world. The antibodies can be customized for the development of different immunoassay formats, including ELISAs, immunohistochemistry, western blot, and so on.
Please feel free to contact us to get more detailed information.
Reference
For Research Use Only.
