Since PK studies are pivotal in new drug discovery, the bioanalytical methods should be well developed and fully validated to ensure the reliability and accuracy of the studies. As a world leader in the industry of drug discovery, Creative Biolabs has extensive experience in in vivo pharmacokinetic method development and validation for a full range of platforms including HPLC, LC/MS/MS, GC/FID or GC/MS, ICP/MS, and various ligand binding assays (ELISA or other cell-based assays).
Method Development and Validation for in vivo PK Studies
Method development and validation is an essential first step to ensure that targeted analytes of interest can be reliably detected and quantified for general sample analysis. When method development is done, the method should be validated to determine its suitability for its intended use, according to FDA, USP, EP or ICH guidelines. To choose the most appropriate analytical methods for the quantitative evaluation of drugs and their metabolites (analytes) and biomarkers are pivotal for the effective and successful conduct of nonclinical and/or biopharmaceutics pharmacology studies. Bioanalytical methods validation involves performing all of the processes which show that a specific method used for quantitative measurement of analytes in a certain biological matrix (e.g., blood, serum, plasma, or urine) is reliable and reproducible for the intended application. General parameters for bioanalytical methods validation include accuracy, precision, selectivity, sensitivity, reproducibility, stability, etc.
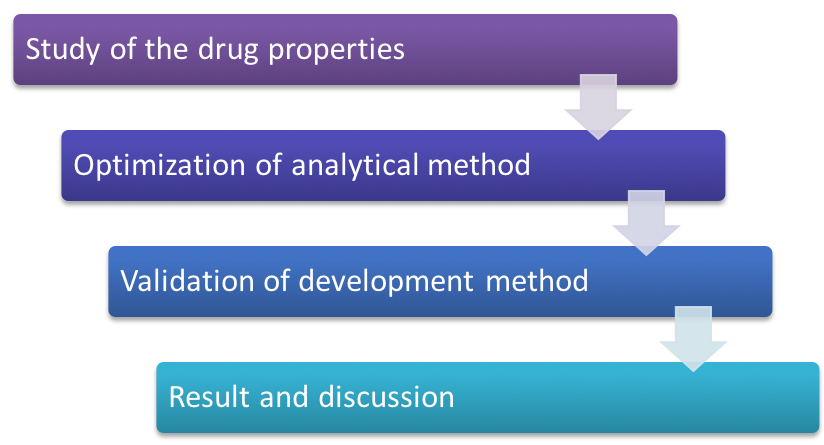
Purpose
Pharmacokinetic sample analysis for large-scale, pre-clinical studies requires a great amount of time, effort, and resources to perform. To maximize efficiency, scientists in Creative Biolabs has launched a whole series of services for method development and validation of in vivo PK studies, and implemented an effective assay strategy requiring minimal staffing and analysis time and cost.
Our Advantages
At Creative Biolabs, everything we do is built around the needs and requirements of our clients all over the world. We're one of the world top CROs specialized in drug discovery, with ability to a matchless range of subject-matter expertise to solve complicated issues.
-
Devoting to offer personalized, responsive service customized for each client.
-
Investigating seriously of your requirements, and develop a proposal which will meet your goals in the most efficient and economical way.
-
Expertise in development of various quantitative assays to support PK studies.
-
Assay validation and analysis of study samples in both GLP and non-GLP environment.
Due to our vast experience with similar projects and refer to the published literature resources, Creative Biolabs can promptly develop the most comprehensive and appropriate method for your research. Moreover, we are available and flexible in providing complete or partial validations, re-validations, and method transfers. The analytical team at Creative Biolabs can provide full documentation including a fully QC/QA reviewed protocol, method SOP, and report for the analyses.
In addition to method development and validation, you might be also interested in other in vivo PK studies listed below:
If you have any special needs for in vivo PK studies at Creative Biolabs, please contact us for more details.
For Research Use Only.