Autoantibody Microarray
With accumulated experience and advanced technologies, Creative Biolabs launches a whole set of natural autoantibodies (NAA) detection and analysis services for our worldwide clients with pleasure. We have established an autoantibody microarray platform, which can help to advance your research projects. These autoantibody microarray detection services can be applied to a wide variety of self-antigens and have been used for screening autoantigen specificities associated with various manifestations of diseases.
Background
Background of Autoantibody
Autoantibodies are a type of antibodies produced by the immune system that are directed against one or more of the individual's own proteins also called autoantigens. Autoantibodies are associated with many autoimmune diseases (notably lupus erythematosus). Normally, autoantibodies can be eliminated by the immune system’s self-regulatory process routinely via the neutralization of autoantibody-producing lymphocytes before they mature. If this process fails, antibodies will react to self-components resulting in diseases occurrence. Autoantibodies elicit body tissues damage through the phagocytosis (ingestion) or lysis (bursting) of healthy cells. Especially the blood cells become the main targets of these actions. For instance, autoimmune hemolytic anemia is caused by autoantibodies binding to red blood cells. In addition, autoantibodies harbor the ability to interfere with the normal cells. For example, in Graves’ disease, autoantibodies bind to receptor cells of the thyroid gland, which stimulates the overproduction of thyroid hormones.
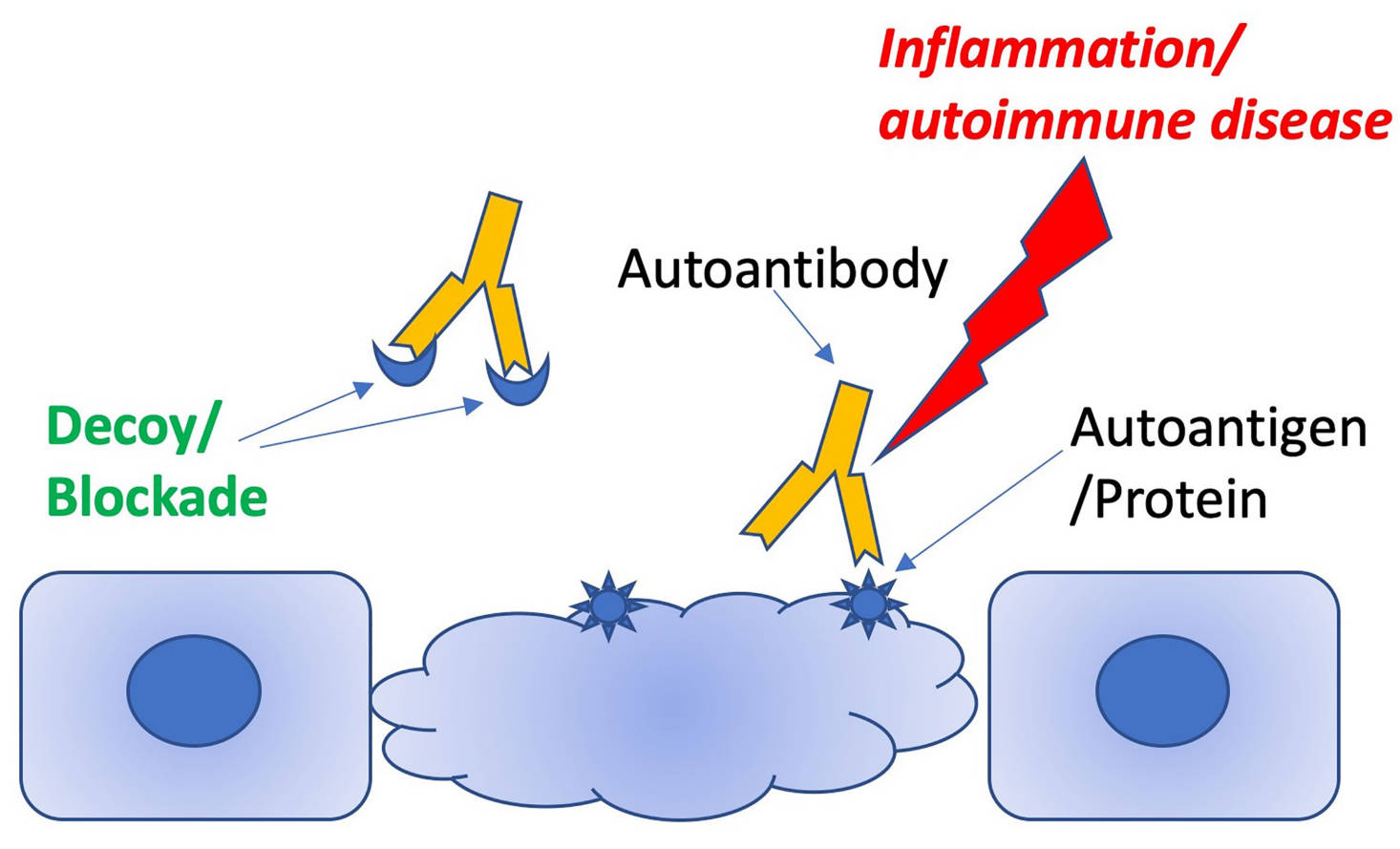 Fig.1 Schematic illustrating how decoy peptides can distract pathogenic antibodies from targeting autoantigens.1
Fig.1 Schematic illustrating how decoy peptides can distract pathogenic antibodies from targeting autoantigens.1
Our Service
Autoantibody Microarray Services
Autoantibodies are associated with various autoimmune diseases and the dysregulation of the immune system. Profiling of autoantibodies against a wide range of autoantigens is a powerful tool for the identification of biomarkers and for prediction and diagnosis of diseases. Currently, the autoantibody microarray can be used to detect the IgG, IgM, IgA and IgE autoantibodies from serum or other body fluids.
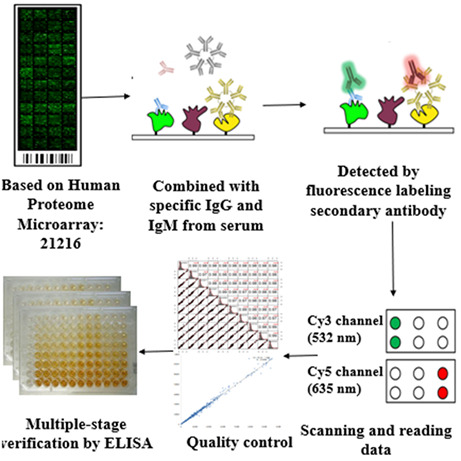 Fig.2 A workflow of using the Human Proteome Microarray to screen autoantibodies to tumor‐associated antigens (TAAbs) to identify serological biomarkers to detect hepatocellular carcinoma.2
Fig.2 A workflow of using the Human Proteome Microarray to screen autoantibodies to tumor‐associated antigens (TAAbs) to identify serological biomarkers to detect hepatocellular carcinoma.2
- Autoantibody Microarray Service for IgM
IgM autoantibody from serum or other body fluids can be detected by using the autoantibody microarray. The samples will be treated with DNAse I, and then incubated with autoantigen array. The autoantibodies binding to the antigens on the array will be detected with labeled anti-IgM and the arrays will be scanned with microarray scanner. The images will be analyzed using software to generate GPR files. The averaged fluorescent intensity (NFI) of each autoantigen will be normalized to internal controls. The final data production will be an Excel spreadsheet containing Non-normalized and Normalized signal intensity as well as the Signal to Noise Ratio (SNR) for all antigens.
- Autoantibody Microarray Service for IgG
IgG autoantibody has 4 subclasses (IgG1, IgG2, IgG3 and IgG4) and each of them may be associated with different clinical manifestations during the disease development. With a high-throughput autoantigen microarray platform, we are able to detect subclasses of IgG autoantibodies against a broad range of autoantigens with high sensitivity and specificity. With this assay, we can simultaneously detect 4 IgG subclasses against various autoantigens on each array. The samples will be treated with DNAse before incubated with autoantigen array. The autoantibodies binding to the antigens on the array will be assayed with biotinylated and IgG secondary antibody and then detected with fluorescent-labeled anti-biotin for imaging. The arrays will be scanned with Microarray Scanner and images will be analyzed using special software to generate GPR files. The averaged fluorescent intensity (NFI) of each autoantigen will be normalized to internal controls.
- Autoantibody Microarray Service for IgA and IgE
IgA and IgE are also important autoantibody subtypes for many autoimmune, infection and allergic diseases. With our high-throughput autoantigen microarray platform, a variety of autoreactive IgA and IgE autoantibodies can be detected with high sensitivity and specificity. In this service, we can simultaneously detect autoreactive IgA and IgE autoantibodies against up to over 100 antigens on each array. The samples will be treated with DNAse I, then incubated with autoantigen array. The autoantibodies binding to the antigens on the array will be detected with biotinylated and IgA and IgE secondary antibody and then detected with fluorescent-labeled anti-biotin for imaging. The arrays will be scanned with Microarray Scanner and images will be analyzed using special software to generate GPR files. The averaged fluorescent intensity (NFI) of each autoantigen will be normalized to internal controls (IgA or IgE).
As a professional service provider in antibody analysis, design, and preparation, Creative Biolabs offers comprehensive NAA services to help you detect NAA-associated immune diseases and new NAA biomarkers using autoantibody microarray in a timely and cost-effective manner. Our tailored services and high-quality products will contribute greatly to the success of your projects. Please contact us for more information and a detailed quote.
Highlight
- High throughput, and multiplicity of the whole workflow.
- High sensitivity, high accuracy, and high specificity.
- Competitive cost performance.
- Short turnaround time.
FAQs
-
Q1: What is the detection range of your autoantibody microarray services?
A: A variety of self-antigens can be detected in our platform, including cell nuclear components, cytoplasmic proteins, phospholipid proteins, cell matrix proteins, mucosal/secreted proteins, glomeruli, and other tissue-specific proteins. -
Q2: What kind of results can I get from this service?
A: You will receive an exhaustive project report and original experiment data within a short turnaround time of 2-3 weeks.
Resource
References
- Cardozo, Timothy, Lila Cardozo, and Mohamed Boutjdir. "Autoantibody: Autoantigen competitor decoys: Application to cardiac phenotypes." Frontiers in Immunology 13 (2022): 812649.
- Yang, Qian, et al. "Human Proteome Microarray identifies autoantibodies to tumor‐associated antigens as serological biomarkers for the diagnosis of hepatocellular carcinoma." Molecular Oncology 17.5 (2023): 887-900.

