Reverse Genetics Technology
Reverse genetics has been an extremely useful tool to study the functions of individual viral genes. It enables researchers to rationally design improved recombinant viruses for use in clinical applications such as vaccination and experimental oncolytic-virus therapy. Especially, reverse genetics has revolutionized the study of RNA viruses.
Introduction of Reverse Genetics
Reverse genetics is opposite to forward genetics. In forward genetics, regular genetics begins with a mutant phenotype, proceeds to show the existence of the relevant gene by progeny ratio analysis, and finally clones and sequences the gene to determine its DNA and protein sequence. However, reverse genetics starts from DNA (cDNA) mutants and helps understand the functions of a gene by analyzing the phenotypic effects caused by genetically engineering specific nucleic acid sequences. Typically, the genetic sequence is changed by directed deletions (gene knockout), insertions (knock-ins), point mutations (site-directed mutagenesis to create null alleles (non-functional)), gene knockdowns (RNAi). Besides, various recombinant oncolytic viruses have been engineered via reverse genetics to improve onco-selectivity, safety, onco-toxicity and stimulation of tumor-specific immunity. Reverse genetics also plays a crucial role in the development of highly specific live attenuated versions of vaccines against specific viruses.
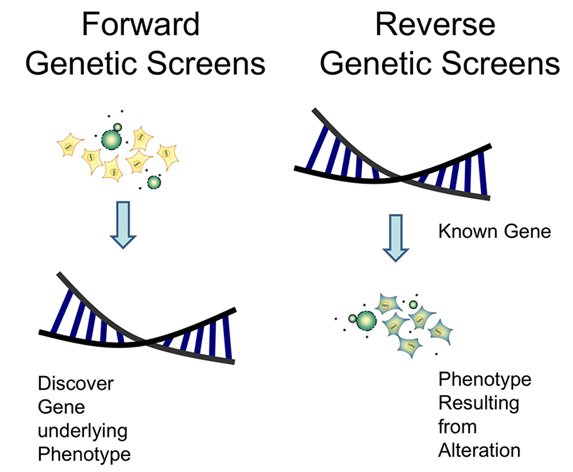 Fig.1 Forward genetics and reverse genetics.
Fig.1 Forward genetics and reverse genetics.
Applications of Reverse Genetics in Oncolytic Virology
Reverse genetics systems are available for virtually all virus families. For DNA viruses (adenovirus, HSV, and vaccinia virus), reverse genetics is a relatively straightforward task because virtually all viral DNA genomes, which can be mutated in vitro, are infectious upon transfection. For RNA viruses, reverse genetics has been one of the most powerful tools to study their biological properties and molecular characteristics. Reverse genetics of RNA viruses involves the manipulation of their genomes at the cDNA level, followed by procedures to produce live infectious progeny virus (wild-type or mutated) after transfection of cDNAs into cells. Recently, plasmid-based reverse genetics technology has been widely applied to optimize the effect of oncolytic RNA virus, such as vesicular stomatitis virus (VSV), Newcastle disease virus (NDV) and measles virus (MV). These recombinant oncolytic viruses can express an exogenous tumor-killing factor, cytokine or chemokine to achieve a good therapeutic effect in clinical trials.
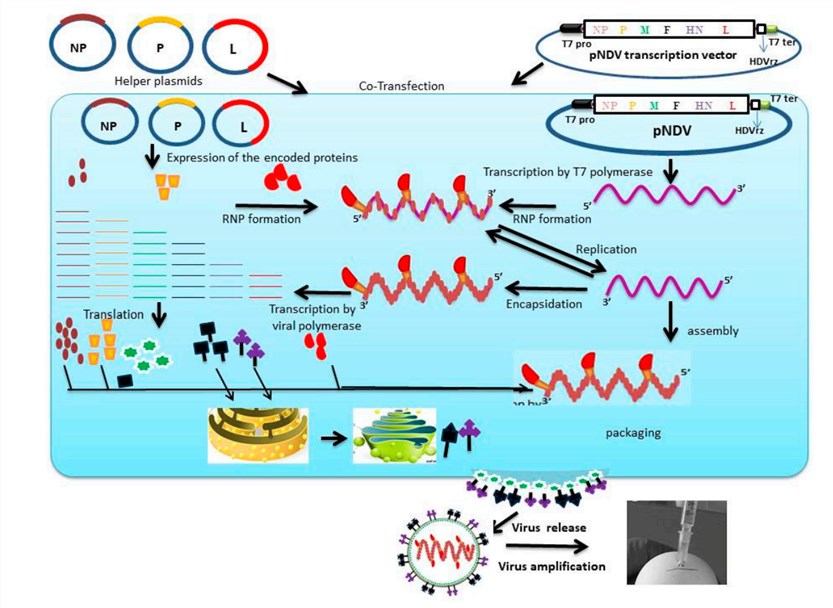 Fig.2 Reverse genetics approach for the generation of engineered Newcastle disease virus in BSR-T7. (Bello, 2020)
Fig.2 Reverse genetics approach for the generation of engineered Newcastle disease virus in BSR-T7. (Bello, 2020)
Reverse Genetics Development at Creative Biolabs
As a leader in oncolytic virus construction, Creative Biolabs has successfully developed a robust reverse-genetics system that can be used to design, construct, and test numerous recombinant oncolytic viruses, which can help us understand basic virus biology, facilitate vaccine development, and aid the development of oncolytic virus therapy. Our plasmid-based reverse genetics system can generate various kinds of infectious viruses with replication kinetics similar to those of wild-type viruses following the transfection of cultured cells.
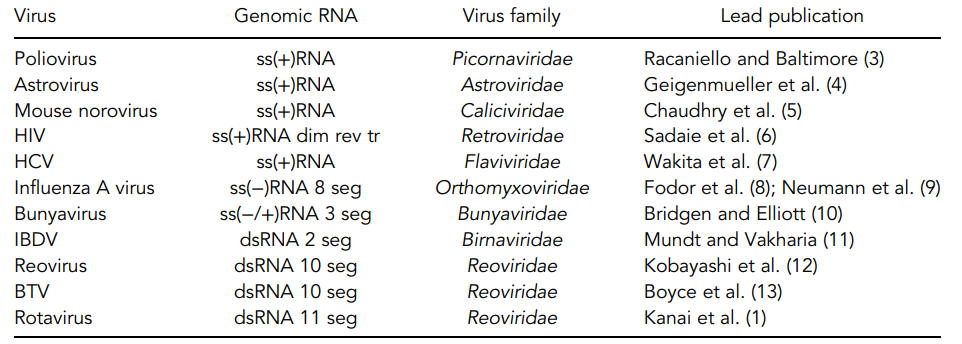 Table.1 Plasmid-only based reverse genetics systems of selected RNA viruses. (Desselberger, 2017)
Table.1 Plasmid-only based reverse genetics systems of selected RNA viruses. (Desselberger, 2017)
Reverse genetics allows for the generation of recombinant viruses or vectors used in functional studies, vaccine development, and oncolytic virus therapy. Our state-of-the-art, plasmid-based reverse genetics approaches provide rapid, convenient, safe and more effective oncolytic viruses.
References
- Bello, M. B.; et al. Exploring the prospects of engineered Newcastle disease virus in modern vaccinology. Viruses. 2020, 12(4): 451.
- Desselberger, U. Reverse genetics of rotavirus. Proceedings of the National Academy of Sciences. 2017, 114(9): 2106-2108.
