Cancer has become one of the leading causes of disease-related death globally. According to updated global cancer statistics from the International Agency for Research on Cancer (IARC), there were nearly 20 million new cancer cases and 9.7 million cancer-related deaths worldwide in 2022.
Chemotherapy and radiation therapy have long been the standard treatments for cancer. However, the lack of tumor specificity in these traditional methods inevitably damages normal tissues, leading to severe adverse reactions. To address the poor specificity of conventional treatments, targeted drug delivery strategies have been developed to selectively deliver cytotoxic drugs to tumor cells, achieving precise killing, significantly reducing systemic toxicity, and improving overall therapeutic outcomes.
The Evolution of Targeted Conjugates
Antibody-Drug Conjugates (ADCs), composed of a monoclonal antibody coupled to a cytotoxic drug via a chemical linker, have become one of the fastest-growing therapeutic categories in oncology. Currently, 15 ADC drugs have received FDA approval, with over 100 more undergoing clinical trials. Following the development of ADCs, Peptide-Drug Conjugates (PDCs) have also gained attention as a next-generation targeted anti-cancer drug.
Beyond monoclonal antibodies and peptides, aptamers—unique targeting ligands—are also being utilized for the conjugation of cytotoxic drugs, leading to the creation of Aptamer-Drug Conjugates (ApDCs). This innovative structure cleverly combines the targeting accuracy of the aptamer with the therapeutic effect of the drug payload.
Aptamers, named by the 2009 Nobel laureate in Physiology or Medicine, Jack Szostak, are short, single-stranded DNA or RNA oligonucleotides that function by forming three-dimensional spatial structures to bind to specific proteins or cells. They offer extensive advantages, including simple synthesis, customizability, good thermal stability, small molecular size, and strong tissue penetration.
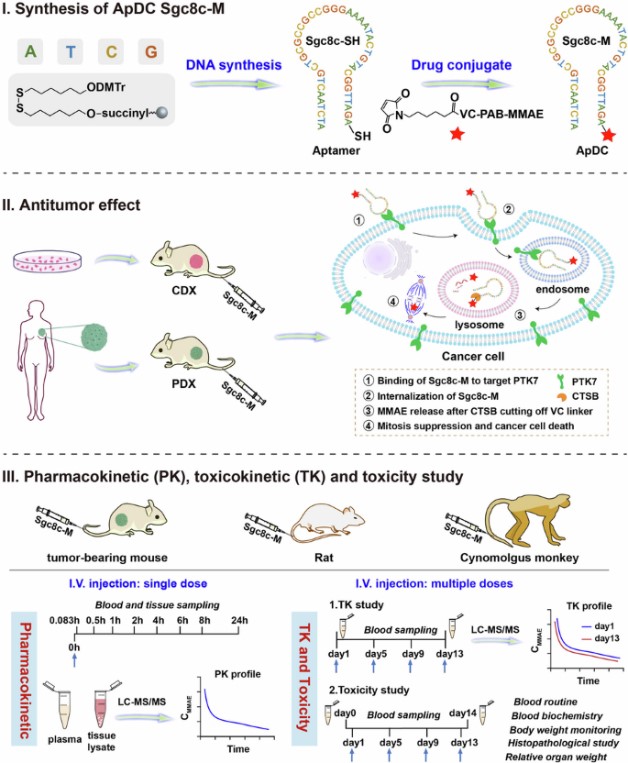
Fig.1 ApDCs offer an attractive strategy for targeted cancer therapy.1
Sgc8c-M: A Promising ApDC for PTK7-Overexpressing Cancers
ApDCs have emerged as a highly attractive new strategy in the field of targeted cancer therapy. In a recent study, Academician Wei-Hong Tan, Research Fellow Xiang-Sheng Liu, and Senior Engineer Jia-Xuan He from the Hangzhou Institute of Medicine, Chinese Academy of Sciences, published a paper titled: An aptamer-drug conjugate for promising cancer therapy with comprehensive evaluation from rodents to non-human primates in the journal Signal Transduction and Targeted Therapy.
The research team developed a Protein Tyrosine Kinase-7 (PTK7)-targeting ApDC, Sgc8c-M. This ApDC drug is formed by conjugating the potent antimitotic agent MMAE with the classic PTK7 aptamer Sgc8c. Sgc8c-M is designed to treat cancers overexpressing PTK7.
Comprehensive Evaluation Highlights Superior Efficacy
Efficacy studies conducted in multiple PTK7-overexpressing cancer types demonstrated that Sgc8c-M effectively induced sustained tumor regression in both cell-line-derived and patient-derived xenograft models. Its effect was shown to be superior to unconjugated MMAE, the chemotherapy drug paclitaxel, and a PTK7-targeting Antibody-Drug Conjugate (ADC).
A comprehensive evaluation, spanning from rodents (mice, rats) to non-human primates (NHP), confirmed that Sgc8c-M is a promising cancer therapeutic.
- Pharmacokinetics (PK) and Toxicokinetics (TK): PK studies in mice showed that Sgc8c-M led to rapid drug accumulation and sustained MMAE levels in tumors, while being quickly cleared from plasma and normal tissues. Further studies in rats confirmed rapid clearance in most organs, with over 75% of MMAE excreted via urine and feces within 24 hours. TK assessment indicated that systemic drug exposure was equivalent after repeated dosing compared to a single dose, with no accumulation.
- Safety and Tolerability in NHP: The team further assessed the PK/TK characteristics and safety of Sgc8c-M in cynomolgus monkeys. Similar to the characteristics observed in rats, Sgc8c-M exhibited good dose-dependent drug exposure and was well-tolerated in cynomolgus monkeys, with no obvious accumulation observed after multiple administrations. Toxicity assessment showed that the highly efficacious therapeutic doses were safe, and toxicity produced by extremely high doses was controllably reversible.
In summary, this research underscores the potential of Sgc8c-M as an effective anti-tumor drug and provides valuable insights for the clinical translation of the emerging ApDC class.
Streamlining Your ADC Development with Creative Biolabs
Creative Biolabs is a leading partner in accelerating antibody-drug conjugate (ADC) development, a crucial strategy in oncology that allows for the selective delivery of cytotoxic agents to cancer cells, minimizing systemic toxicity.
We provide a comprehensive, modular suite of services to guide your ADC program from discovery through preclinical evaluation:
- Antibody Discovery: Identifying high-affinity, specific antibodies optimized for targeted conjugation.
- DrugLnk™ Custom Linker-Payload Synthesis: Utilizing our proprietary DrugLnk™ platform to synthesize custom linkers and potent cytotoxic payloads, ensuring optimal stability and targeted release.
- Antibody Design & Conjugation: Expert services in antibody engineering and site-specific conjugation to ensure high-quality, consistent conjugate synthesis.
- ADC In Vitro Analysis: Rigorous testing, including stability, binding, internalization, and cytotoxicity assays, to verify the functional integrity of your ADC.
- ADC In Vivo Analysis: Providing robust Pharmacokinetic (PK), Toxicokinetic (TK), safety, and efficacy studies in relevant animal models to facilitate preclinical success.
Partner with Creative Biolabs to leverage our expertise and technology, ensuring an efficient and successful pathway to the next generation of targeted therapy.
Reference
- Su, Minhui, et al. “An aptamer-drug conjugate for promising cancer therapy with comprehensive evaluation from rodents to non-human primates.” Signal Transduction and Targeted Therapy 10.1 (2025): 316. CC BY4.0. https://doi.org/10.1038/s41392-025-02399-1