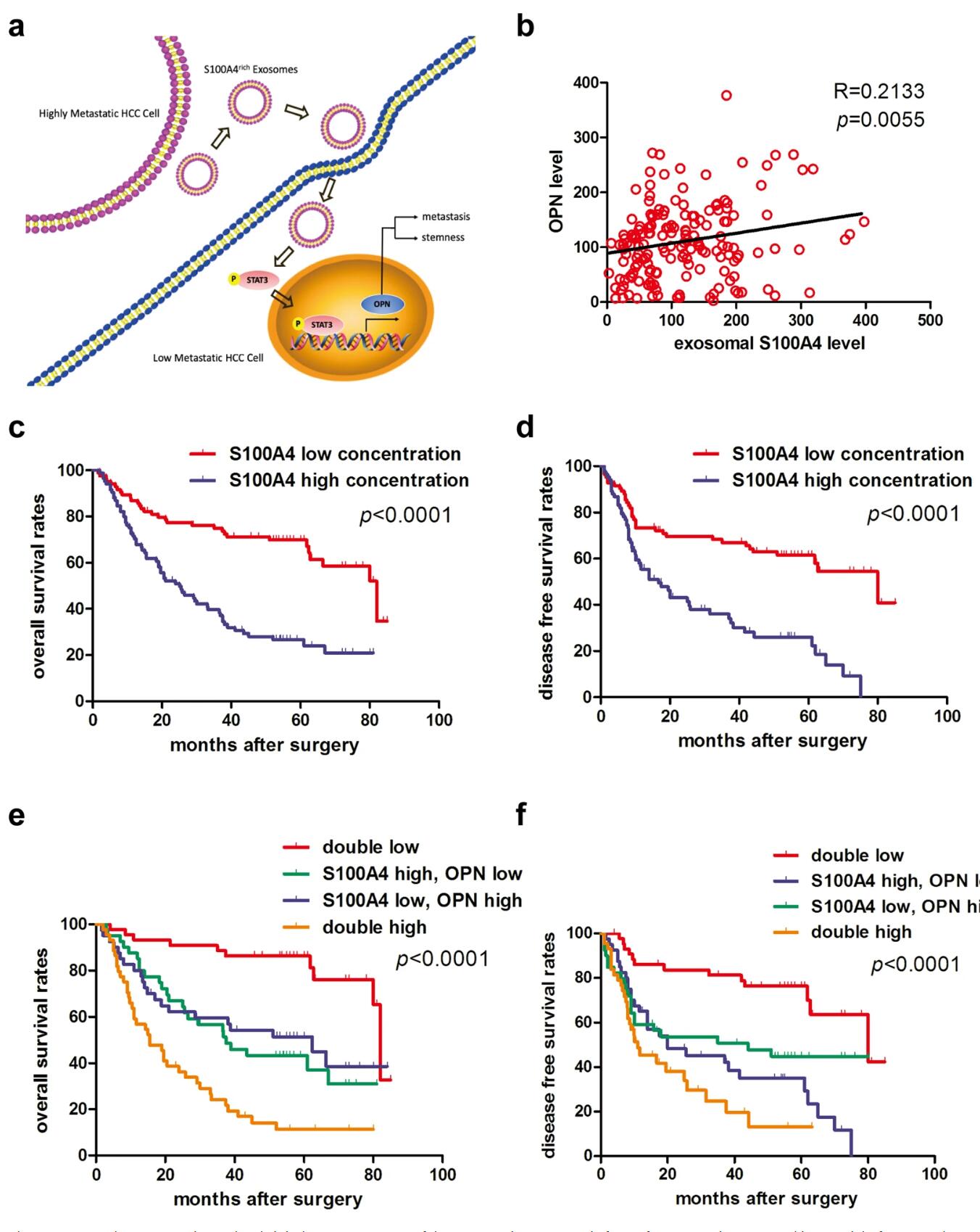
Intercellular communication plays a crucial role in tumor progression and metastasis. However, the mechanisms by which tumor cells interact via exosomes remain incompletely understood. Recently, researchers performed proteomic profiling of exosomes derived from hepatocellular carcinoma (HCC) cells with different metastatic potentials and identified S100A4 in exosomes from highly metastatic cells. They found that exosomal S100A4 enhanced the stemness and metastatic potential of low-metastatic cells. This study was published in Signal Transduction and Targeted Therapy.
 Tumor metastasis and recurrence are responsible for 90% of cancer-related deaths, representing the most significant features of malignant tumors. More than half of hepatocellular carcinoma (HCC) patients experience tumor recurrence within five years of surgical resection, which is a major cause of poor prognosis in HCC. Intratumoral heterogeneity contributes to drug resistance and post-treatment tumor relapse. Therefore, understanding the intratumoral heterogeneity of HCC and the key drivers of metastatic potential is essential.
Tumor metastasis and recurrence are responsible for 90% of cancer-related deaths, representing the most significant features of malignant tumors. More than half of hepatocellular carcinoma (HCC) patients experience tumor recurrence within five years of surgical resection, which is a major cause of poor prognosis in HCC. Intratumoral heterogeneity contributes to drug resistance and post-treatment tumor relapse. Therefore, understanding the intratumoral heterogeneity of HCC and the key drivers of metastatic potential is essential.
Intercellular communication plays a vital role in tumor progression and metastasis. Classical communication methods include direct cell-cell contact and the secretion of soluble molecules, such as growth factors and cytokines. In recent years, exosomes have emerged as an effective system for intercellular signaling, functioning in both local and distant organs, and have been identified as key players in cancer metastasis. These lipid bilayer-enclosed nanovesicles contain diverse components, including proteins, lipids, and nucleic acids, and originate from intracellular multivesicular bodies. Exosome-mediated communication has been widely recognized as a powerful promoter of tumor invasion. Studies have shown that glioblastoma cells with the EGFRvIII mutation, known for high invasiveness, can transfer mutant EGFRvIII protein via exosomes, significantly enhancing the malignancy of wild-type EGFRvIII cells. Similarly, exosomes from highly metastatic melanoma cells can enhance the malignancy of bone marrow progenitor cells. However, the role of exosome-mediated communication between HCC cells with differing metastatic potentials remains poorly understood.
S100 calcium-binding protein A4 (S100A4), a member of the S100 family, plays a crucial role in tumor metastasis by regulating adhesion, extracellular matrix remodeling, and cell motility. In liver cancer, mesenchymal stem cell-secreted S100A4 promotes invasiveness via the miR155-SOCS1-MMP9 pathway. Moreover, exosomes from bladder cancer upregulate S100A4 in urothelial cells. Additionally, S100A4 regulates the formation of the pre-metastatic niche mediated by pancreatic cancer-derived exosomes.
In this study, researchers explored the functional role of exosomes in tumor metastasis and their prognostic value in HCC. They extracted exosomes from HCC cells with varying metastatic potentials and evaluated the pro-metastatic effects of exosomes derived from highly metastatic HCC cells (HMH) were evaluated both in vitro and in vivo. Exosomal proteins were identified using iTRAQ-based proteomics and validated through cell line analysis, xenograft tumor models, and functional assays.
Exosomes released by HMH significantly enhanced the invasiveness of low-metastatic HCC (LMH) cells in vitro and promoted metastasis in vivo. S100A4 was identified as a functional factor in HMH-derived exosomes. Compared to controls or exosomes with low S100A4 expression, S100A4-enriched exosomes significantly promoted tumor metastasis both in vitro and in vivo. Additionally, exosomal S100A4 induced the expression of osteopontin (OPN) and other metastasis/stemness-related genes. Exosomal S100A4 activated OPN transcription via STAT3 phosphorylation.
HCC patients with high plasma S100A4 levels also had worse prognoses. In summary, exosomes from HMH cells enhance the metastatic potential of LMH cells, with exosomal S100A4 acting as a key metastasis enhancer by activating STAT3 phosphorylation and upregulating OPN expression. This indicates that exosomal S100A4 is a novel prognostic marker and therapeutic target for HCC metastasis.
Reference:
Sun, Haoting et al. “Exosomal S100A4 derived from highly metastatic hepatocellular carcinoma cells promotes metastasis by activating STAT3.” Signal Transduction and Targeted Therapy vol. 6,1 187. 26 May. 2021, doi:10.1038/s41392-021-00579-3
Related Services:
