Hello, fellow science enthusiasts! Today, we’re diving into the fascinating world of cancer therapeutics, where the quest for precision and efficacy is ever-evolving. Groundbreaking research has introduced a novel approach to tackle tumor heterogeneity, a key obstacle in effective cancer treatment. Let’s explore how scientists are developing smart nanoplatforms to degrade specific proteins within cancer cells, paving the way for more precise and powerful therapies.
The Challenge: Tumor Heterogeneity
Tumor heterogeneity, in simple terms, refers to the diversity of cells within a tumor. It’s like a gang of criminals where not everyone has the same weakness. Some cells are rapidly dividing, others are dormant, and some are resistant to conventional treatments. This diversity makes it incredibly difficult to wipe out the entire tumor population, often leading to relapse and treatment failure.
One particularly challenging group of cells within a tumor is the cancer stem-like cells (CSCs). Think of them as the ‘bosses’ of the tumor. They have the ability to self-renew and drive tumor growth, and they’re notoriously resistant to standard chemotherapy and radiotherapy.
To make matters even more complex, tumors aren’t uniform environments. They have regions with good blood supply and plenty of oxygen (normoxic), and other regions that are starved of oxygen (hypoxic). CSCs tend to hang out in these hypoxic regions, adding another layer of complexity to treatment.
The Solution: Protein Degrader Nanoplatforms
Scientists are now developing innovative strategies to overcome tumor heterogeneity. One promising approach involves the use of protein degraders. Protein degraders are like guided missiles that can selectively degrade specific proteins within cells.
A protein degrader molecule is designed to bind to both the protein you want to destroy (the target protein) and another protein that tags the target for degradation. The cell’s own machinery then breaks down the unwanted protein.
However, simply injecting protein degraders into the body has limitations. They may not accumulate effectively in the tumor, and they can cause off-target effects, meaning they degrade the protein in healthy cells as well.
To address these issues, researchers are developing protein degrader nanoplatforms. These are essentially tiny packages that carry the protein degraders and are designed to release their cargo specifically within the tumor.
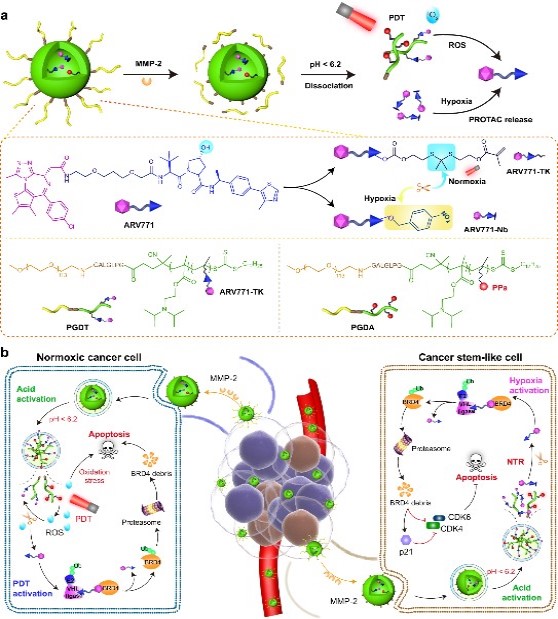
Fig. 1 Schematic diagram of the protein degrader nano platform.1
Smart Nanoparticles for Targeted Delivery
The key to these nanoplatforms is their ability to respond to specific cues within the tumor environment. By engineering nanoparticles that are sensitive to certain conditions, scientists can ensure that the protein degraders are released precisely where they’re needed.
In a recent study, researchers designed a nanoplatform that combines two types of protein degraders:
- ROS-activatable protein degraders: These are released in response to reactive oxygen species (ROS), which can be generated by photodynamic therapy (PDT) in well-oxygenated regions of the tumor.
- Hypoxia-responsive protein degraders: These are activated in the oxygen-starved (hypoxic) regions where CSCs tend to reside.
The nanoplatform itself is designed to be sensitive to matrix metalloproteinase-2 (MMP-2), an enzyme that is often overexpressed in tumors. This sensitivity allows the nanoparticles to accumulate and penetrate deeply into the tumor tissue.
The Study: A Multi-Pronged Attack on Tumors
In the study, researchers used this smart nanoplatform to target a protein called BRD4, which plays a crucial role in cancer growth. By degrading BRD4 in both normoxic and hypoxic regions, they aimed to eliminate both the bulk of the tumor cells and the stubborn CSCs.
Here’s a breakdown of how the system works:
- Targeted Accumulation: The nanoparticles are injected into the bloodstream and accumulate in the tumor due to their size and the leaky nature of tumor blood vessels (the EPR effect).
- MMP-2 Triggered Release: Once in the tumor, the nanoparticles encounter MMP-2, which triggers the removal of a protective coating, enhancing their penetration into the tumor tissue.
- ROS-Mediated Protein Degrader Activation: In the normoxic regions, photodynamic therapy (PDT) is used to generate ROS. This triggers the release of the ROS-activatable protein degraders, which then degrade BRD4.
- Hypoxia-Driven Protein Degrader Activation: In the hypoxic regions, the hypoxia-responsive protein degraders are activated, also leading to the degradation of BRD4.
Promising Results: In Vitro and In Vivo
The researchers conducted a series of experiments, both in cell cultures (in vitro) and in animal models (in vivo), to test the efficacy of their protein degrader nanoplatform.
The results were highly encouraging:
- The nanoplatform effectively degraded BRD4 in both normoxic and hypoxic environments.
- The growth of breast and head-neck tumors was significantly inhibited in animal models.
- The treatment led to a reduction in the number of CSCs, which is crucial for preventing tumor relapse.
The Significance: A New Frontier in Cancer Therapy
This research represents a significant advancement in the development of targeted cancer therapies. By creating a nanoplatform that can respond to the specific microenvironments within a tumor, scientists have taken a major step towards overcoming the challenge of tumor heterogeneity.
This innovative approach holds the potential to:
- Improve the efficacy of cancer treatment
- Reduce off-target effects and toxicity
- Prevent tumor relapse
While this research is still in its early stages, it offers a glimpse into the future of cancer therapy, where treatments are precisely tailored to the unique characteristics of each tumor.
Looking Ahead: The Future of Precision Medicine
The development of smart nanoplatforms is a rapidly evolving field. Scientists are constantly exploring new ways to improve their targeting capabilities and to incorporate additional therapeutic agents.
In the future, we may see nanoplatforms that can:
- Respond to even more specific tumor markers
- Deliver multiple drugs or therapies simultaneously
- Be activated by external stimuli, such as ultrasound or magnetic fields
The possibilities are truly exciting, and this research brings us closer to a future where cancer can be treated with unprecedented precision and effectiveness.
Creative Biolabs offers comprehensive protein degraders molecule discovery services.
Protein Degraders Molecule Design and Discovery
- Protein Degraders Molecule Discovery: Utilizes computational tools and chemical libraries to design novel protein degraders, aiming to recruit target proteins to E3 ligases for degradation.
- Ligand Design for Target Protein: Employs structure-based design to create high-affinity ligands for target proteins, key to specific degradation.
- Ligand Screening for E3 Ligase: Uses high-throughput methods to identify E3 ligase ligands, optimizing protein degraders’ degradation ability.
- Linker Design and Optimization: Focuses on linker properties to enhance protein degraders stability, permeability, and efficiency.
protein degraders Evaluation and Testing
- Protein Degraders In Vitro Evaluation: Assesses protein degraders binding, target degradation in cell-free and cell-based assays.
- Protein Degraders In Vivo Animal Test: Evaluates protein degraders efficacy, safety, and pharmacokinetics in animal models for clinical development.
References
- Gao, Jing, et al. “A region-confined PROTEIN DEGRADERS nanoplatform for spatiotemporally tunable protein degradation and enhanced cancer therapy.” Nature Communications1 (2024): 6608. https://doi.org/10.1038/s41467-024-50735-w
