In the ever-evolving landscape of biomedical research, targeted protein degradation (TPD) has emerged as a transformative approach with the potential to redefine disease treatment. By selectively eliminating pathogenic proteins, this technology addresses limitations of traditional therapies, particularly for “undruggable” targets. This blog explores the latest advancements in protein degraders, their mechanisms, applications, and the future of this exciting field, drawing insights from a recent review published in Biomarker Research.
Understanding Targeted Protein Degradation
Proteins play central roles in cellular processes, and their dysregulation often drives diseases like cancer, neurodegeneration, and autoimmune disorders. Traditional drug discovery focuses on inhibiting protein function, but TPD offers a distinct advantage: it removes the problematic protein entirely. This is achieved through two primary mechanisms: the ubiquitin-proteasome system (UPS) and autophagy.
UPS-Dependent Degradation
The UPS is a highly regulated pathway where proteins tagged with ubiquitin chains are degraded by the proteasome. Proteolysis-targeting chimeras are small molecules that exploit this system. They consist of two moieties: one that binds the target protein and another that recruits an E3 ubiquitin ligase. This brings the target into proximity with the ligase, leading to ubiquitination and subsequent proteasomal degradation.
Autophagy-Mediated Degradation
Autophagy is a cellular recycling process that engulfs and degrades damaged proteins or organelles. AUTACs (Autophagy-Targeting Chimeras) redirect autophagic machinery to specific proteins. By linking a target-binding ligand to a lysosome-targeting moiety, AUTACs induce autophagosome formation and degradation of the target.
Diverse Classes of Protein Degraders
Protein degraders have evolved beyond proteolysis-targeting chimeras and AUTACs, with several innovative classes gaining traction.
Molecular Glues
These small molecules stabilize protein-protein interactions, often between E3 ligases and targets not naturally recognized by the ligase. For example, thalidomide derivatives recruit cereblon (CRBN) to degrade IKZF1/3, offering therapeutic benefits in multiple myeloma.
VIPER-TACs
A recent innovation, VIPER-TACs leverage viral E3 ligases to enhance target specificity. By repurposing viral machinery, these degraders can achieve tissue-specific protein degradation, as demonstrated in breast cancer models where viral ligases selectively degraded BRD4 and MST1/2.
Engineered Platelets
In a groundbreaking study, engineered platelets were used as carriers for protein degraders. By conjugating HSP90 inhibitors to platelet surfaces, researchers achieved targeted degradation of BRD4 in breast cancer cells, reducing tumor recurrence and metastasis.
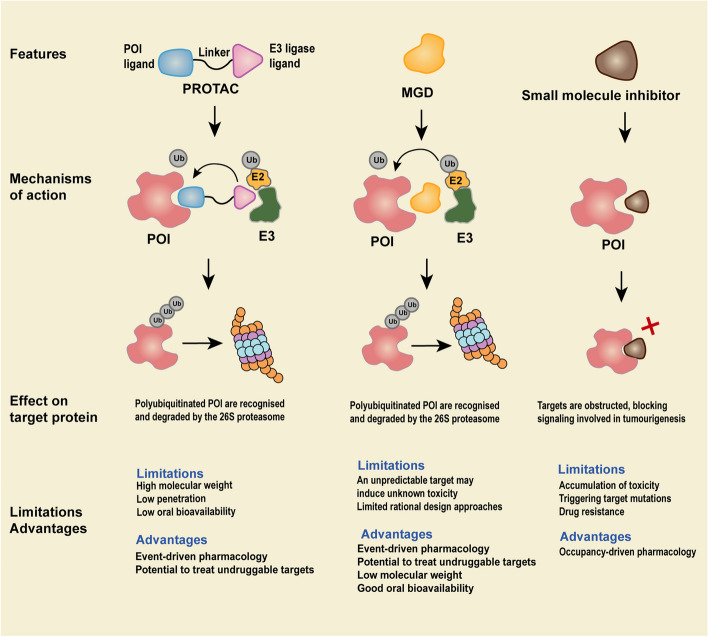
Fig.1 Understanding targeted protein degradation and small molecule inhibitor action.1
Applications Across Diseases
The versatility of protein degraders has been demonstrated in various therapeutic areas.
Oncology
- Proteolysis-targeting chimeras targeting androgen receptors (AR) and estrogen receptors (ER) have shown promise in hormone-dependent cancers. For instance, ARV-471, an ER-targeting proteolysis-targeting chimera, is in Phase III trials for breast cancer.
- Molecular glues like lenalidomide have revolutionized multiple myeloma treatment by degrading IKZF1/3, disrupting oncogenic pathways.
Immunology
Degraders targeting IRAK4, a key mediator of inflammatory signaling, are advancing in clinical trials. For example, LT-002, an IRAK4 degrader, is being tested in patients with atopic dermatitis and hidradenitis suppurativa.
Neurodegeneration
TPD strategies are being explored to clear aggregation-prone proteins like α-synuclein in Parkinson’s disease. Recent studies using brain-penetrant proteolysis-targeting chimeras have shown efficacy in preclinical models.
Challenges and Future Directions
While protein degraders offer immense potential, several hurdles must be addressed.
Target Specificity
Off-target effects remain a concern. Advances in computational design, such as the ProteinMPNN algorithm, are improving degrader specificity by optimizing binding interfaces.
Delivery Systems
Poor bioavailability and limited tissue penetration hinder systemic delivery. Nanoparticle formulations and cell-based carriers, like engineered platelets, are being explored to overcome these challenges.
Clinical Translation
Scaling up production and ensuring reproducibility are critical. Regulatory frameworks, such as FDA’s accelerated approval pathways, are supporting the translation of degraders into clinical practice.
Combination Therapies
Degraders are increasingly being paired with immunotherapies or chemotherapy. For example, combining BRD4 degraders with PD-1 inhibitors enhances anti-tumor immunity in preclinical models.
Emerging Trends in 2025
The field of protein degradation is advancing rapidly, with several notable developments in 2025:
- Molecular Glues for Solid Tumors: A novel HuR-targeting molecular glue, DEG6498, entered clinical trials for colorectal and lung cancers, marking a milestone for targeting RNA-binding proteins.
- AI-Driven Design: Machine learning models like ProteinMPNN are accelerating degrader development by predicting optimal sequences for stability and binding affinity.
- Cellular Carriers: Engineered platelets and exosomes are being repurposed as delivery vehicles, offering site-specific degradation.
- Regulatory Support: The FDA’s approval of DEG6498 highlights growing recognition of TPD’s therapeutic potential, with more IND applications expected in 2025.
Targeted protein degradation is reshaping the future of medicine by offering precise, efficient, and durable solutions for previously incurable diseases. From proteolysis-targeting chimeras to molecular glues and viral-based degraders, the field continues to push boundaries. As technology evolves and clinical data accumulate, protein degraders are poised to become a cornerstone of personalized therapy.
Creative Biolabs offers comprehensive protein degrader services via cutting-edge technologies for targeted agent discovery, evaluation, and validation. Specializing in proteolysis-targeting chimeras, the company provides end-to-end solutions from molecule design to in vivo testing for challenging therapeutic targets.
Categorized Service Descriptions
- Protein Degrader Molecule Discovery: Focuses on rational design and screening of small-molecule degraders, including target validation, linker optimization, and proteolysis-targeting chimera synthesis for E3 ligase engagement.
- Protein Degrader In Vitro Evaluation: Provides in vitro assays for degrader potency/selectivity, including degradation kinetics, ubiquitination profiling, and cytotoxicity testing.
- Protein Degrader In Vivo Animal Test: Conducts preclinical animal studies for efficacy/safety, including PK profiling, target engagement, tumor regression, and toxicity assessments.
References
- Feng, Yupiao, Xinting Hu, and Xin Wang. “Targeted protein degradation in hematologic malignancies: clinical progression towards novel therapeutics.” Biomarker Research 12.1 (2024): 85. https://doi.org/10.1186/s40364-024-00638-1
