A Critical Need for Innovation in Global Health
Tuberculosis (TB) remains a formidable foe in global health, consistently ranking as the leading cause of death from an infectious disease worldwide. The existing Bacillus Calmette-Guérin (BCG) vaccine, while effective against severe childhood disease, exhibits widely varying efficacy that notably declines in adolescents and adults. With high rates of BCG vaccination prevalent in endemic areas, any next-generation vaccine must be designed not just to replace, but to effectively augment and exceed the protection of BCG.
The urgent global health priority to develop an improved TB vaccine faces a monumental hurdle: the systematic selection of protective antigens. The Mycobacterium tuberculosis (M. tuberculosis) genome contains approximately 4,000 open reading frames (ORFs), offering an overwhelming repertoire of potential candidates.
A groundbreaking study by Vidal et al. (2025), “Mining the CD4 antigen repertoire for next-generation tuberculosis vaccines,” offers a meticulously systematic solution to this challenge. It outlines the discovery and development of a novel trivalent messenger RNA-lipid nanoparticle (mRNA-LNP) vaccine designed specifically to combat M. tuberculosis by systematically leveraging the human CD4 T cell antigen repertoire.
The Systematic Antigen Selection Strategy
Historically, many vaccine candidates were selected primarily based on their immunogenicity—the ability to elicit a robust immune response. However, the study confirms that antigen immunogenicity is necessary but not sufficient for protective efficacy against TB. This critical insight drove the research team to pioneer an in vivo screening pipeline that prioritized genuine protective efficacy.
The key to this strategy lay in focusing the initial screen on antigens that are already established targets of CD4 T cells in humans with latent TB (LTB), a clinical state where adaptive immunity successfully controls the infection.
Key Steps in Antigen Discovery:
- Repertoire Mining: The screen began with 36 top CD4 T cell antigen targets identified in LTB individuals, along with 6 antigens already in clinical development, utilizing a DNA vaccine platform for efficient initial screening in mice.
- Efficacy-Based Ranking: Protective efficacy was rigorously measured as the fold reduction in lung bacterial load following M. tuberculosis aerosol challenge in a mouse model (CB6F1 strain) This process identified a spectrum of protection, highlighting eight lead antigens showing $\ge 2.5$-fold protection. Importantly, most of these top protective antigens, including the three ultimately selected, are novel and had not yet been evaluated in clinical vaccine trials.
- Phylogenetic Clustering and Cross-Reactivity: The top protective antigens naturally segregated into phylogenetic clusters with distinct structural and functional properties, specifically belonging to the Proline-Proline-Glutamic acid (PPE), Type VII secretion (Esx), Proline-Glutamic acid (PE), and Antigen 85 (Ag85) families. Critical to the design, antigens within these clusters showed significant immunologic cross-reactivity among their CD4 T cell epitopes.
Defining the Trivalent mRNA-LNP Vaccine
Based on the compelling data demonstrating efficacy and cross-reactivity, the development focused on selecting the most protective antigen from three different phylogenetic clusters to ensure broad and robust coverage.
The chosen candidates for the novel vaccine were:
- Rv1387 (PPE20): A member of the PPE family.
- Rv0287 (EsxG): A member of the critical Esx family of Type VII secretion proteins.
- Rv1788 (PE18): A member of the PE family.
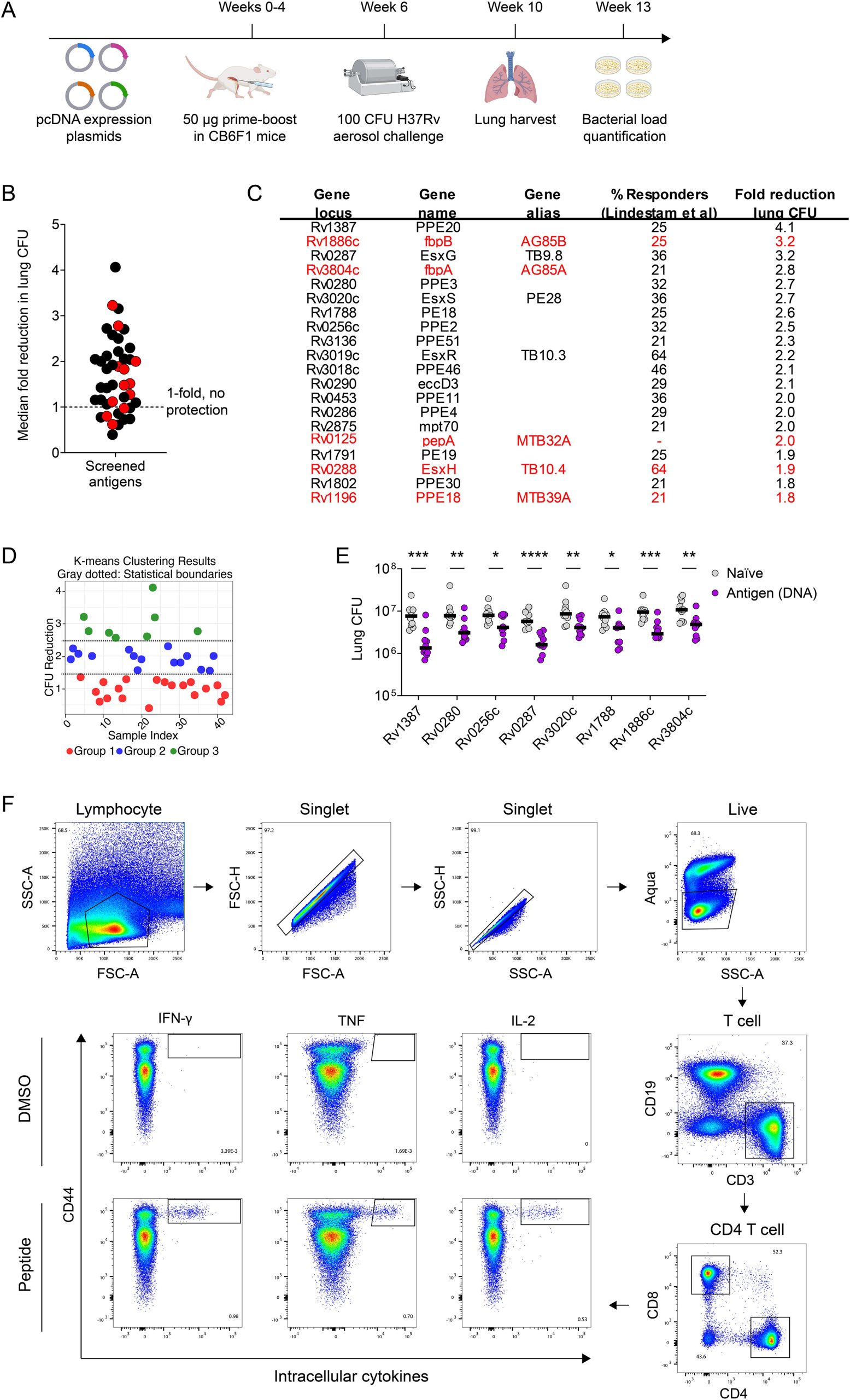
Fig.1 In vivo screen, DNA vaccine results, and flow cytometry for novel TB vaccine antigen discovery.1
The Superiority of the mRNA-LNP Platform
The choice of the mRNA-LNP platform proved instrumental to the vaccine’s success, capitalizing on its flexibility, scalability, and potent immunogenicity observed during the COVID-19 pandemic.
Comparative studies in mice showed a clear advantage for the mRNA-LNP delivery platform over the initial DNA platform:
- Enhanced Efficacy: The monovalent mRNA-LNP vaccines achieved greater reductions in lung CFU (ranging from 4.2- to 7.2-fold) compared to their DNA vaccine counterparts, demonstrating a statistically significant improvement.
- Optimal T Cell Phenotype: The mRNA-LNP platform induced a superior CD4 T cell response phenotype, primarily characterized by substantially higher percentages of IL-2-secreting cells, a response previously associated with increased TB protection.
When multiplexed into a fixed-dose cocktail, the trivalent combination (Rv1387, Rv0287, and Rv1788) demonstrated robust performance, yielding an 8.2-fold reduction of lung bacterial loads in the standard mouse challenge model, suggesting a synergistic effect of the antigen combination.
Augmenting and Exceeding BCG Protection
A key requirement for any new TB vaccine is its effectiveness when administered to a population already vaccinated with BCG. The trivalent mRNA-LNP vaccine not only proved potent on its own but also showed the capability to dramatically boost existing BCG immunity in the standard high-dose challenge model:
- Delayed Boosting: BCG priming followed by a delayed trivalent mRNA-LNP boost resulted in a 63-fold reduction in bacterial loads, significantly exceeding the 14-fold reduction observed with BCG alone.
- Co-administration: Simultaneous administration of BCG and the trivalent mRNA-LNP vaccine achieved a 73-fold reduction in bacterial loads, demonstrating superior protective immunity (compared to 21-fold for BCG alone).
Protection Against Low-Dose Challenge and Dissemination
Perhaps most compelling are the results from the low-dose challenge model (1 MID50), which is considered to better recapitulate the dynamics of human M. tuberculosis transmission. In this more stringent model, the combination of BCG and trivalent mRNA-LNP demonstrated superior prevention of infection and disease dissemination:
- The infection rate was reduced to 26% (from 60% in naive mice).
- The rate of bilateral lung dissemination was eliminated entirely (0% vs. 56% in naive mice).
- Both vaccine groups achieved a massive reduction in median lung bacterial loads by >25,000-fold compared with naive mice.
High Recognition in Humans: Translational Relevance
The research concluded by validating the clinical relevance of the chosen antigens. Cellular immune responses were assessed in an adolescent cohort in South Africa with pre-existing exposure to M. tuberculosis (IGRA-positive).
The results confirmed that the trivalent antigen combination elicits a broad and high rate of recognition:
- 84% of participants recognized any antigen in the trivalent mRNA-LNP vaccine.
- 68% recognized at least two antigens.
This coverage rate significantly surpassed that of a leading clinical candidate (M72/AS01E), highlighting the potential of this novel combination to confer robust, clinically relevant protective immunity in human populations already exposed to the pathogen.
A Novel Strategy for TB Vaccine Development
The systematic process of mining the human CD4 T cell repertoire, validating efficacy through rigorous in vivo screening, and leveraging the potent mRNA-LNP platform has led to the definition of a clear next-generation TB vaccine concept. The trivalent mRNA-LNP vaccine (Rv1387, Rv0287, Rv1788) has been shown to effectively augment and exceed BCG protection against infection, dissemination, and bacterial burden in preclinical models. This defined vaccine concept is currently advancing toward Phase 1 clinical trials.
At Creative Biolabs, we accelerate your vaccine development with end-to-end expertise. Our core services feature sophisticated DNA & RNA vaccine design, utilizing the cutting-edge mRNA vaccine platform for enhanced immunogenicity and scalability. We streamline the crucial initial phase with advanced antigen prediction and screening/discovery, ensuring the selection of highly protective candidates for next-generation vaccines
Reference
- Vidal, Samuel J., et al. “Mining the CD4 antigen repertoire for next-generation tuberculosis vaccines.” Cell(2025). CC BY4.0. https://doi.org/10.1016/j.cell.2025.08.027
