“We have the drugs, but the parasite is learning faster than we are.”
This blunt warning from a parasitology lab in Northeast India sums up the quiet crisis surrounding Fasciolopsis buski—a flatworm so large it is nick-named the “giant intestinal fluke.” Roughly 10 million people, most of them children in South and Southeast Asia, carry the parasite. Many never notice it, yet severe infections can trigger intestinal bleeding, protein-losing enteropathy, and even death. The standard pill—praziquantel—still works, but scattered reports of reduced efficacy have researchers worried. Meanwhile, no vaccine has ever reached a human or pig trial.
That may soon change. A team from India has just published the first epitope-based vaccine blueprint for fasciolopsiasis built entirely with computers (Frontiers in Genetics, 2024). Here is what the study means for clinicians, public-health workers, and anyone who wonders why parasite vaccines lag decades behind those for viruses.
The Forgotten Trematode Makes a Comeback
Fasciolopsiasis looks like a disease of the past—black-and-white photos of rice-farmers with swollen bellies. Yet recent surveys in Uttar Pradesh, India, and central Vietnam keep finding new clusters. The parasite completes its life cycle in freshwater snails and aquatic plants such as water chestnuts. Children often peel the raw plants with their teeth, releasing the encysted larvae. Pigs amplify the transmission, acting as living reservoirs. Climate change is expanding the snail’s range northward, and urban markets now ship water spinach from endemic villages to non-endemic cities. The stage is set for silent outbreaks.
Why Drugs Alone May Not Win the Long Game
Praziquantel is cheap, safe, and donated by the millions of tablets. But three red flags keep parasitologists awake:
- Single-dose pressure favors any worm carrying mutations that reduce drug uptake.
- Mass drug administration in schools treats the child but does not stop reinfection a week later from the same pond.
- No new drug classes for intestinal trematodes are in the pipeline.
The World Health Organization’s 2030 roadmap therefore lists “vaccines for zoonotic helminths” as a high-priority research gap.
Mining the Parasite’s Own Cookbook
Rather than hunting blindly for vaccine antigens, the Indian team started with omics. They had previously sequenced the adult-stage transcriptome of F. buski and identified 9 proteins that sit at the host-parasite interface: tegument (skin), gut lining, and excretory-secretory vesicles. These proteins are the parasite’s Swiss-army knives—digesting host tissues, detoxifying bile, and even manipulating the immune response.
| Protein | Main Job in the Worm | Why It Makes a Good Vaccine Target |
| Thioredoxin-glutathione reductase (TGR) | Keeps oxidative stress in check | Without it, the worm drowns in its own ROS |
| Cathepsin B & Cathepsin L | Meat-grinder proteases that let the worm tunnel through gut wall | Blocking them = mechanical paralysis |
| Leucine aminopeptidase (LAP) | Final step in protein digestion | Highly abundant in gut, exposed to host antibodies |
| Fatty-acid-binding protein (FABP3) | Imports host lipids | Essential in a lipid-poor intestinal environment |
| 14-3-3 epsilon | Signalling scaffold | Disrupts worm’s ability to respond to host signals |
| Glutathione S-transferase (GST) | Detoxifies bile acids | Immunogenic in other flukes |
| Tegumental calcium-binding EF-hand proteins 3 & 4 | Sense calcium, shape tegument | Altering calcium flux can destabilize surface membranes |
From Protein Lists to Epitope Maps: How the Computer Took Over
Traditional vaccine discovery would grind each protein in the lab, inject animals, and wait. The new study flips the script using immunoinformatics:
- B-cell epitopes (9-mers) were predicted with ABCPred.
- Helper T-cell epitopes (HTL) were filtered for strong binding to the most common human and pig MHC-II alleles.
- Cytotoxic T-cell epitopes (CTL) were chosen for supertypes A2, A3, B7, covering >88 % of global populations.
- Toxic, cross-reactive, or human-like epitopes were discarded.
- The remaining 45 epitopes were stitched into a single 901-amino-acid chimeric protein with molecular linkers (KK, AAY, GPGPG) to ensure each segment folds correctly.
To light the immunological fuse, the team added a TLR2-agonist adjuvant (lipoprotein LprA) at the N-terminus. Toll-like receptor 2 is the innate sensor that shouts “danger!” to dendritic cells.
Stress-Test in Silico: Does the Construct Hold Up?
Before a pipette is lifted, the virtual vaccine passed four checkpoints:
- Structure prediction (I-TASSER) gave a compact, soluble protein.
- Molecular docking to TLR2 showed a binding energy of –1,287 kJ mol⁻¹—stronger than many licensed adjuvants.
- 150-ns molecular dynamics in water revealed a stable RMSD of 0.84 nm and 963 hydrogen bonds holding the complex together.
- Immune simulation (C-IMMSIM) predicted robust IgG1/IgG2 responses after three injections, with memory B and T cells ready for a secondary challenge.
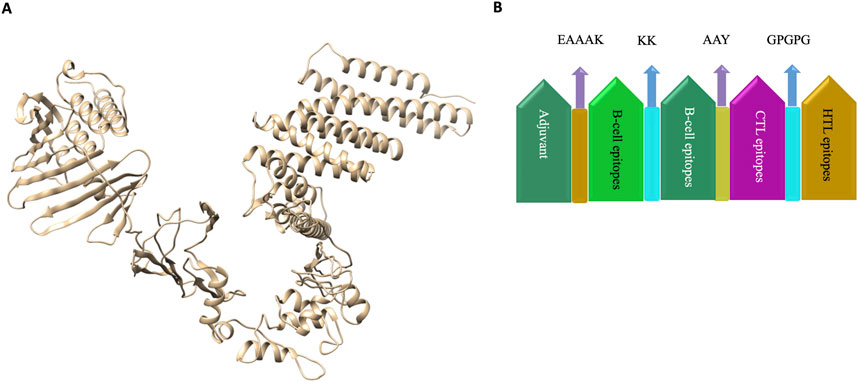
Fig. 1 Predicted 3-D structure of the chimeric vaccine. 1
From Bytes to Barnyards: What Happens Next?
The authors inserted the codon-optimized gene into the standard pET28a(+) plasmid for E. coli expression—an off-the-shelf system that keeps costs low. The next hurdles are:
- Pilot expression to confirm soluble protein at gram scale.
- Mouse immunogenicity to verify neutralizing antibodies against native parasite proteins.
- Pig challenge trials, because porcine reservoirs drive human transmission. A 40–60 % reduction in worm burden in pigs could slash environmental egg load within one farming season.
Lessons for the Broader Vaccine World
The study is more than a one-off for an obscure worm. It showcases a platform approach:
- Multi-omics (transcriptome + pathway mapping) pinpoints druggable or vaccinable nodes.
- Epitope down-selection shrinks the antigen from whole protein to 15-mer peptides, cutting manufacturing costs.
- Adjuvant rationalization uses innate-receptor biology instead of empirical oil-in-water emulsions.
The same workflow is now being applied to Fasciola hepatica (liver fluke) and Opisthorchis viverrini (bile-duct fluke). A pan-trematode cocktail is no longer fantasy.
The Clock Is Ticking—But So Is the Tech
Artificial intelligence is accelerating every layer: AlphaFold2 offers near-experimental structures; AlphaFold-Multimer predicts how adjuvant and antigen dance together; and generative diffusion models can now dream up linkers that maximize epitope exposure while minimizing aggregation. The F. buski study is the latest proof-of-concept that dry-lab speed can outpace wet-lab caution—if regulators and funders keep up.
Take-Home for Clinicians and Health Educators
- Counsel families in endemic zones: cook aquatic plants, but also support local pig vaccination campaigns once products become available.
- Monitor for praziquantel non-response—prolonged egg shedding after treatment may signal resistance.
- Advocate for One Health budgets that link human clinics, veterinary services, and environmental engineers. A fluke that jumps from pig to child to pond will not be defeated by tablets alone.
Looking Ahead
The giant intestinal fluke has thrived for centuries because it exploits gaps between human behavior, farming practice, and policy. An epitope-based vaccine will not replace sanitation or snail control, yet it offers the missing biological shield—one that can bend the transmission curve downward even when the world’s ponds remain contaminated. For the first time, we have a detailed blueprint; now the real race begins at the bench, the barn, and the back-yard garden where water spinach grows.
Creative Biolabs offers a range of professional vaccine design services to address various infectious disease challenges, including parasitic vaccines and in silico vaccine design.
Parasitic Vaccine Design Service: This service focuses on developing high-quality vaccines for preventing parasitic diseases, which pose significant threats to animal health and productivity. It covers different types of parasitic vaccines such as live vaccines, killed vaccines, subunit and recombinant vaccines.
In Silico Vaccine Design Services: Leveraging computer-aided informatics and high-throughput technologies, this service uses in silico models and databases to predict and design new vaccines and their components. It is cost-effective, time-saving, applicable for high throughput, and helps increase the chances of vaccine success.
Reference
Konhar, Ruchishree, et al. “In silico design of an epitope-based vaccine ensemble for fasliolopsiasis.” Frontiers in Genetics 15 (2025): 1451853. https://doi.org/10.3389/fgene.2024.1451853.
